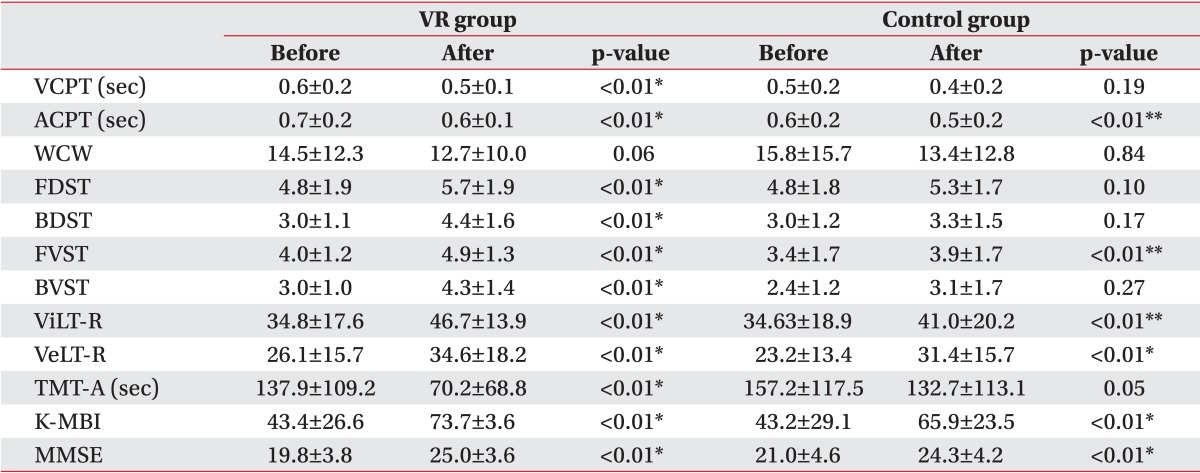
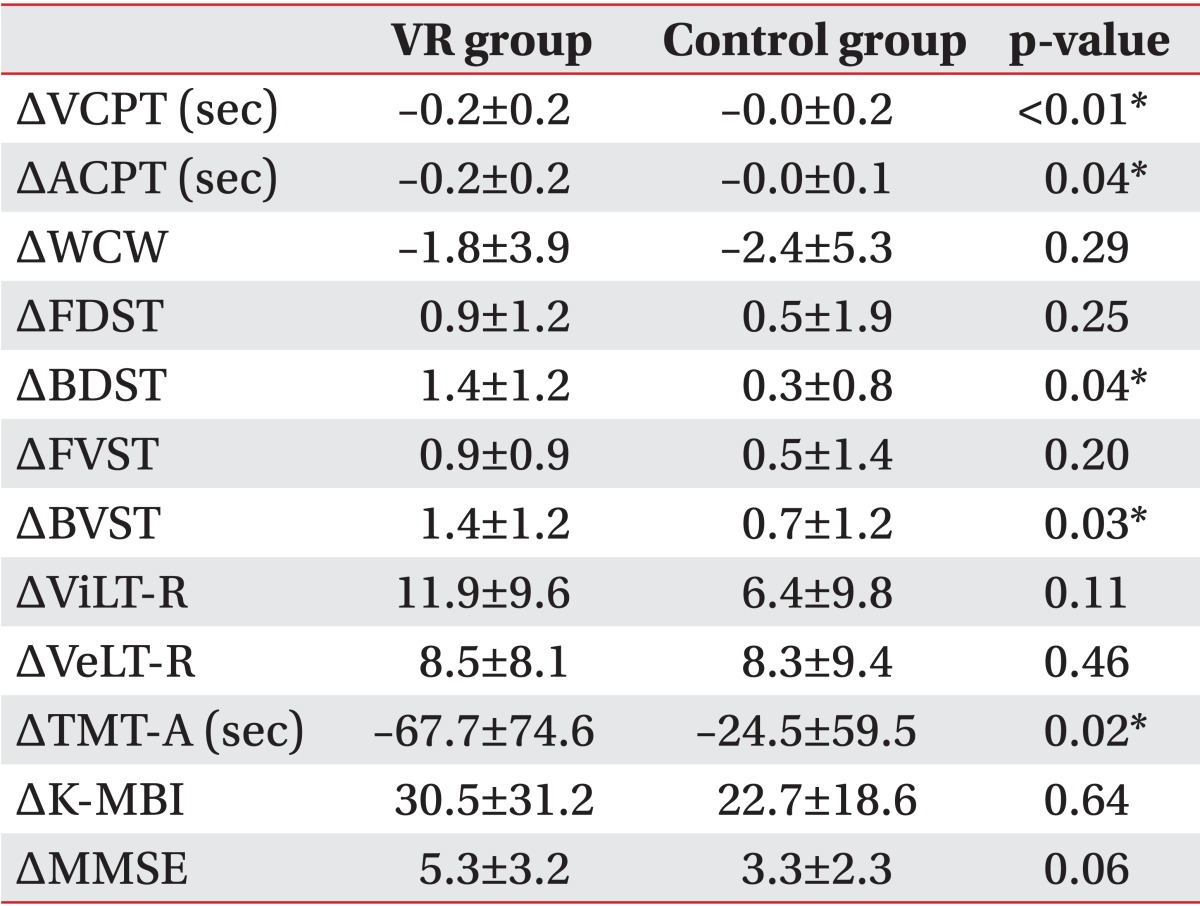
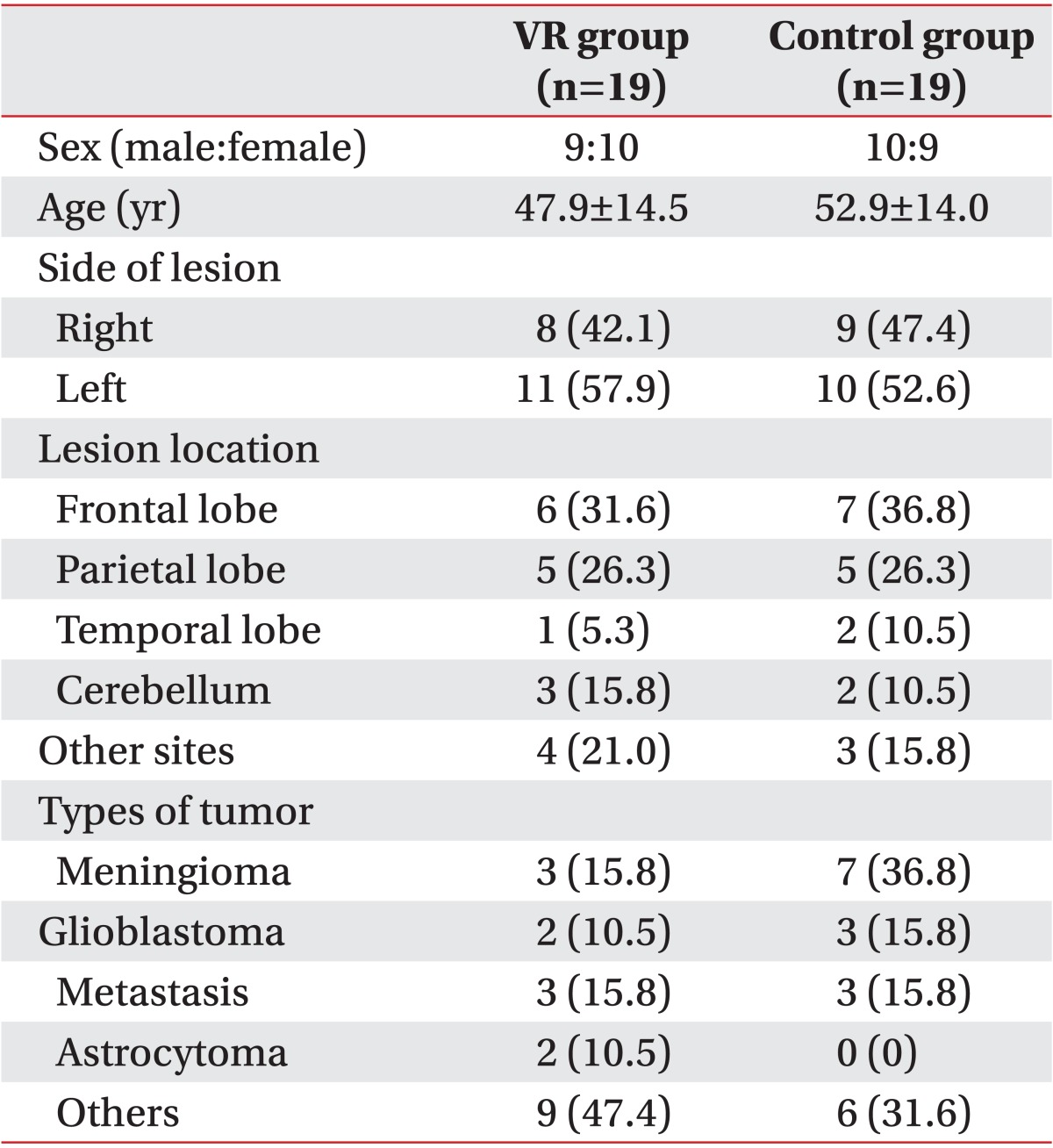
1. Gehring K, Sitskoorn MM, Aaronson NK, Taphoorn MJ. Interventions for cognitive deficits in adults with brain tumours. Lancet Neurol 2008;7:548-560. PMID:
18485318.


2. Shaw EG, Rosdhal R, D'Agostino RB Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol 2006;24:1415-1420. PMID:
16549835.


4. dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, Guedes da Silva K, Oliveira Tde P, Peterson Zomignani A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson's disease: effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy 2012;98:217-223. PMID:
22898578.


5. Cherniack EP. Not just fun and games: applications of virtual reality in the identification and rehabilitation of cognitive disorders of the elderly. Disabil Rehabil Assist Technol 2011;6:283-289. PMID:
21158520.


6. Yip BC, Man DW. Virtual reality-based prospective memory training program for people with acquired brain injury. NeuroRehabilitation 2013;32:103-115. PMID:
23422463.


7. Hanten G, Cook L, Orsten K, Chapman SB, Li X, Wilde EA, et al. Effects of traumatic brain injury on a virtual reality social problem solving task and relations to cortical thickness in adolescence. Neuropsychologia 2011;49:486-497. PMID:
21147137.


9. Hofmann M, Rosler A, Schwarz W, Muller-Spahn F, Krauchi K, Hock C, et al. Interactive computer-training as a therapeutic tool in Alzheimer's disease. Compr Psychiatry 2003;44:213-219. PMID:
12764709.


11. Werner P, Rabinowitz S, Klinger E, Korczyn AD, Josman N. Use of the virtual action planning supermarket for the diagnosis of mild cognitive impairment: a preliminary study. Dement Geriatr Cogn Disord 2009;27:301-309. PMID:
19252401.


12. Lee SJ, Chun MH. Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch Phys Med Rehabil 2014;95:431-438. PMID:
24239790.


13. Kim J, Kim K, Kim DY, Chang WH, Park CI, Ohn SH, et al. Virtual environment training system for rehabilitation of stroke patients with unilateral neglect: crossing the virtual street. Cyberpsychol Behav 2007;10:7-15. PMID:
17305443.


14. Kim BR, Chun MH, Kim LS, Park JY. Effect of virtual reality on cognition in stroke patients. Ann Rehabil Med 2011;35:450-459. PMID:
22506159.



15. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol 2004;3:159-168. PMID:
14980531.


16. Weitzner MA, Meyers CA. Cognitive functioning and quality of life in malignant glioma patients: a review of the literature. Psychooncology 1997;6:169-177. PMID:
9313282.


17. Anderson SW, Damasio H, Tranel D. Neuropsychological impairments associated with lesions caused by tumor or stroke. Arch Neurol 1990;47:397-405. PMID:
2322133.


18. Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 2004;22:157-165. PMID:
14701778.


19. Holden MK. Virtual environments for motor rehabilitation: review. Cyberpsychol Behav 2005;8:187-219. PMID:
15971970.


20. Zelinski EM, Reyes R. Cognitive benefits of computer games for older adults. Gerontechnology 2009;8:220-235. PMID:
25126043.



21. Sveistrup H. Motor rehabilitation using virtual reality. J Neuroeng Rehabil 2004;1:10PMID:
15679945.



22. Kizony R, Raz L, Katz N, Weingarden H, Weiss PL. Video-capture virtual reality system for patients with paraplegic spinal cord injury. J Rehabil Res Dev 2005;42:595-608. PMID:
16586185.


23. You SH, Jang SH, Kim YH, Hallett M, Ahn SH, Kwon YH, et al. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter-blind randomized study. Stroke 2005;36:1166-1171. PMID:
15890990.


24. Yong Joo L, Soon Yin T, Xu D, Thia E, Pei Fen C, Kuah CW, et al. A feasibility study using interactive commercial off-the-shelf computer gaming in upper limb rehabilitation in patients after stroke. J Rehabil Med 2010;42:437-441. PMID:
20544153.


25. Kim MY, Lee KS, Choi JS, Kim HB, Park CI. Effectiveness of cognitive training based on virtual reality for the elderly. J Korean Acad Rehabil Med 2005;29:424-433.
26. Sanchez-Vives MV, Slater M. From presence to consciousness through virtual reality. Nat Rev Neurosci 2005;6:332-339. PMID:
15803164.



27. Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch Phys Med Rehabil 1999;80:661-667. PMID:
10378492.