Effects of Botulinum Toxin-A for Spasticity and Nociceptive Pain in Individuals With Spinal Cord Injury: A Systematic Review and Meta-Analysis
Article information
Abstract
We conducted a systematic review and meta-analysis to examine the protective effects of botulinum toxin-A (Botox-A) on spasticity and nociceptive pain in individuals with spinal cord injuries (SCIs). PubMed, Embase, and Cochrane Library databases were searched from inception to July 2023. The primary outcome of interest was spasticity and nociceptive pain. We pooled the available data using the generic inverse variance method, and we used a fixed-effect/random-effects model. We then calculated standardized mean difference (SMD) and 95% confidence intervals (95% CIs) to estimate the effect size. A total of fourteen studies meeting the inclusion criteria comprised two randomized controlled trials, five pre-post studies, and seven case reports. Across the various study designs, the majority of trials were assessed to have fair to high quality. The meta-analysis shows that Botox-A significantly decreased spasticity (SMD, -1.73; 95% CI, -2.51 to -0.95; p<0.0001, I2=48%) and nociceptive pain (SMD, -1.79; 95% CI, -2.67 to -0.91; p<0.0001, I2=0%) in SCI patients. Furthermore, Botox-A intervention improved motor function, activities of daily living (ADL), and quality of life. Our study suggests that Botox-A may alleviate spasticity and nociceptive pain in SCI patients. Moreover, the observed improvements in motor function, ADL, and overall quality of life following Botox-A intervention underscore its pivotal role in enhancing patient outcomes.
INTRODUCTION
Spasticity and pain frequently emerge as secondary sequelae following spinal cord injury (SCI), affecting around 60%–80% of cases [1]. Spasticity is also associated with a decline in quality of life (QoL) and increased mortality rates in SCI patients [2]. Pain following SCI, whether nociceptive or neuropathic [3], is equally prevalent and severe, adversely affecting physical, psychological, and social well-being, often worsening alongside other pain types. In the latest systematic review, nociceptive pain was found in 45% of cases, while neuropathic pain occurred in 58% of cases [4].
Despite significant advancements in managing spasticity and pain, addressing the fundamental barriers to QoL in individuals with SCI remains challenging. The management of spasticity often necessitates a multidisciplinary approach involving anti-spasticity medications, physical therapy, and, in some cases, surgical interventions [5]. However, oral anti-spasticity medications commonly prescribed to SCI patients are associated with various systemic adverse effects, such as sedation, confusion, hallucinations, nausea, and liver toxicity [6]. Compounding the issue, a significant portion of spasticity patients, around 40%, cannot tolerate the side effects of oral anti-spasticity agents [7], and physiotherapy frequently yields inconsistent outcomes in this population [8]. Local injections of botulinum toxin-A (Botox-A) offer a promising alternative to mitigate the side effects of oral anti-spasticity drugs and address the limitations of physiotherapies in SCI patients [9,10].
Botox-A, derived from the Clostridium botulinum bacterium, exerts its potent action by proteolytically cleaving synaptosomal associated protein-25 at nerve endings, thus impeding the release of acetylcholine neurotransmitter from axon terminals, resulting in muscle relaxation [11]. Numerous studies have demonstrated the safety and efficacy of Botox-A in managing spasticity across various neurological conditions including stroke, and traumatic brain injury [12,13]. Nearly a decade ago, a systematic review assessed the efficacy of Botox-A in managing spasticity among SCI patients [14]; however, given the dynamic nature of this field and the increasing number of published studies, the findings from this review may be outdated, reflecting the evolving landscape and highlighting the need for updated evidence to inform current clinical practice. Notably, recent randomized controlled trials (RCTs), such as the study by Yan et al. [15], have begun to evaluate the safety and efficacy of Botox-A specifically in SCI patients. Botox-A has been used to reduce spasticity in the rehabilitation setting, and its potential to manage pain is increasingly popular in the last decades [16,17]. A recent clinical review was conducted to evaluate the therapeutic efficacy of Botox-A for neuropathic pain in individuals with SCI [17]. Nonetheless, the therapeutic potential of Botox-A on nociceptive pain in SCI patients remains uncertain.
This systematic review and meta-analysis explore existing evidence on the protective role of Botox-A against spasticity and nociceptive pain in SCI patients. The evidence presented underscores the therapeutic potential of alleviating spasticity and nociceptive pain to enhance QoL in individuals with SCI.
METHODS
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The PRISMA checklist is provided in Supplementary Material.
Search strategy and data sources
We searched PubMed, Embase, and Cochrane Library electronic databases to identify all relevant trials up to July 2023. The reference lists of the included studies and of relevant reviews were examined for additional relevant trials. An electronic search of three databases was conducted, using the following search terms: “spinal cord injuries,” “botulinum toxin,” and “muscle spasticity.” No limits (e.g., on language or publication date) were used. The in-depth search strategy performed in major electronic databases is given in Supplementary Table S1.
Inclusion criteria and outcomes of interest
Population
Any SCI patients who were diagnosed with spasticity will be included.
Interventions
Trials that evaluated the effects of Botox-A on spasticity in patients with SCI. The combined therapy of Botox-A with other interventions will be excluded. In terms of RCT, all participants in the control group underwent any therapies for spasticity, but not any types of Botox-A. In pre-post design studies, before Botox-A injection were categorized as “pre” (control arm), and after Botox-A treatment were deemed “post” (experimental).
Outcomes
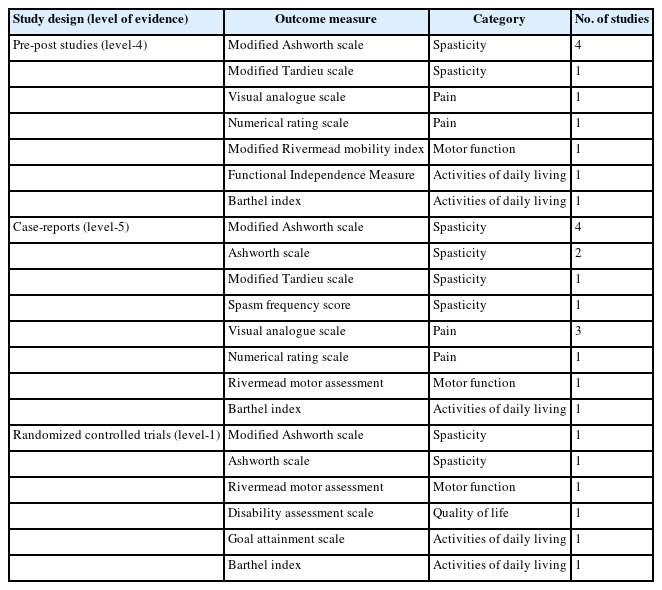
The primary outcome encompasses spasticity, assessed through validated scales including the modified Ashworth scale (MAS), modified Tardieu scale (MTS), Ashworth scale (AS), and spasm frequency score (SFS), as well as nociceptive pain measured by the visual analogue scale (VAS) and numerical rating scale (NRS). Nociceptive pain being defined by the diagnostic criteria proposed by the international SCI pain classification [18]. The secondary outcome includes the evaluation of activities of daily living (ADL) through tools such as the Barthel index (BI), Functional Independence Measure (FIM), or goal attainment scale (GAS). Additionally, motor function is assessed using the modified Rivermead mobility index or other pertinent tools, and QoL is measured using the disability assessment scale (DAS).
Study design
This study includes RCTs, before and after studies (pre-post design), or case report studies that assess the efficacy of Botox-A for the treatment of spasticity in patients with SCI. Any other studies, such as animal studies, conference abstracts, observational studies, letters, and reviews were removed.
Selection of studies
We used reference management software (Mendeley) to organize and manage a large number of citations, as well as remove duplicate articles. The remaining unique articles were then imported into a Rayyan web application to allocate the references randomly (https://www.rayyan.ai/). Based on titles and abstracts screening, two independent reviewers identified all of the pertinent articles, and trials that did not meet the inclusion criteria were eliminated. The same reviewers who were responsible for the primary screening had also evaluated the selected full-text articles for final inclusion. Any disagreements in study selection or data extraction were resolved by discussion between the reviewers, if consensus had not been reached, a third reviewer arbitrated.
Data extraction
Two reviewers individually extracted the following information: first author name and publication year, location, study design, sample size, patient characteristics, interventions, and outcomes measured. With regard to the pre-post study, the mean and standard deviation (SD) of final scores and the total number of participants before Botox-A treatment and after Botox-A treatment were extracted. Finally, we contacted the corresponding author via ResearchGate to retrieve any missing data. If efforts to obtain the raw data were unsuccessful, the article was removed from the meta-analysis. When the data were presented graphically, GetData Graph Digitizer [19] was used to extract numerical data from graphs or figures.
Quality assessment
Two reviewers individually evaluated the methodological quality of the selected trials. We applied the National Institutes of Health quality assessment tool for all pre-post design studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Additionally, the methodological quality of RCT was appraised by two authors using the Physiotherapy Reference Database (PEDro) scale, which has been designed to explore the reliability of data and provides a score out of 1–10 [20]. Studies with a PEDro score of 6–8, 4–5, and scoring below 4 are categorized as good quality, fair quality, and poor quality respectively [20]. Finally, the methodological quality of the selected case studies had evaluated utilizing the tool proposed by Murad et al. [21] to assess case series and case reports. This tool employs four domains for assessment: (1) selection, (2) ascertainment, (3) causality, and (4) reporting. If a trial has sufficient data in a domain, it was given one point. A total score of 4 out of 4 was considered high quality, 3 out of 4 was considered moderate quality, and a score ≤2 was considered low quality. Both the high and moderate-quality trials had considered having enough data to make inferences related to clinical practice. Finally, the Grading of Recommended Assessment, Development and Evaluation (GRADE) approach was used to determine the certainty of evidence for the primary outcomes (https://www.gradeworkinggroup.org/).
Data analysis
The variation of control groups between the included two RCTs, a formal meta-analysis was not possible. Furthermore, our included case reports were heterogeneous and did not include a control group. Hence, quantitatively synthesizing the results of each case study and estimating effect size is not possible either. Therefore, only descriptive and narrative results of qualitative analysis on the role of “Botox-A” described in each individual study were provided. However, statistical analysis was carried out by Review Manager software (RevMan 5.3; The Nordic Cochrane Centre) for pre-post design studies. We enumerated standardized mean differences (SMDs) for continuous data where trials used different scales for each outcome [22]. For each study and each comparison, we calculated the SMD, the pooled SD, and the standard error of the SMD. Where the correlation coefficient was unable to be extracted from publications or raw data, a conservative value of 0.5 was assumed [22]. This can be considered a conservative estimate when using the change scores from the baseline. When SD was not reported we calculated SD from the t-value or the p-value [23], otherwise we inputted the highest SD from the existing meta-analysis. We pooled the available data using the generic inverse variance method, and we used a random-effects model. In the absence of clinical or statistical heterogeneity, we also applied a fixed-effect model for pooling. To assess the statistical heterogeneity, we performed a chi2 test and calculated the I2 value according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions. The I2 test was utilized to assess heterogeneity among the studies (I2=25%, low heterogeneity; I2=50%, moderate heterogeneity; and I2=75%, high heterogeneity) [24]. The fixed-effects model was used for the meta-analysis when I2 was ≤50% and the random-effects model when I2 was >50% [25,26]. Finally, we performed sensitivity analyses to evaluate the effect of the correlation coefficient; one assuming no correlation (when correlation coefficient=0.00) and one assuming a higher correlation (when correlation coefficient=0.80). And, p-value <0.05 is considered statistically significant. When conducting a meta-analysis, it's generally recommended to assess publication bias through funnel plot asymmetry only if there are at least 10 studies included. This is because with fewer studies, the tests lack sufficient power to differentiate chance from actual asymmetry. Since our study included fewer than 10 studies, evaluating publication bias through funnel plot asymmetry and Egger regression was not possible.
RESULTS
Search strategy
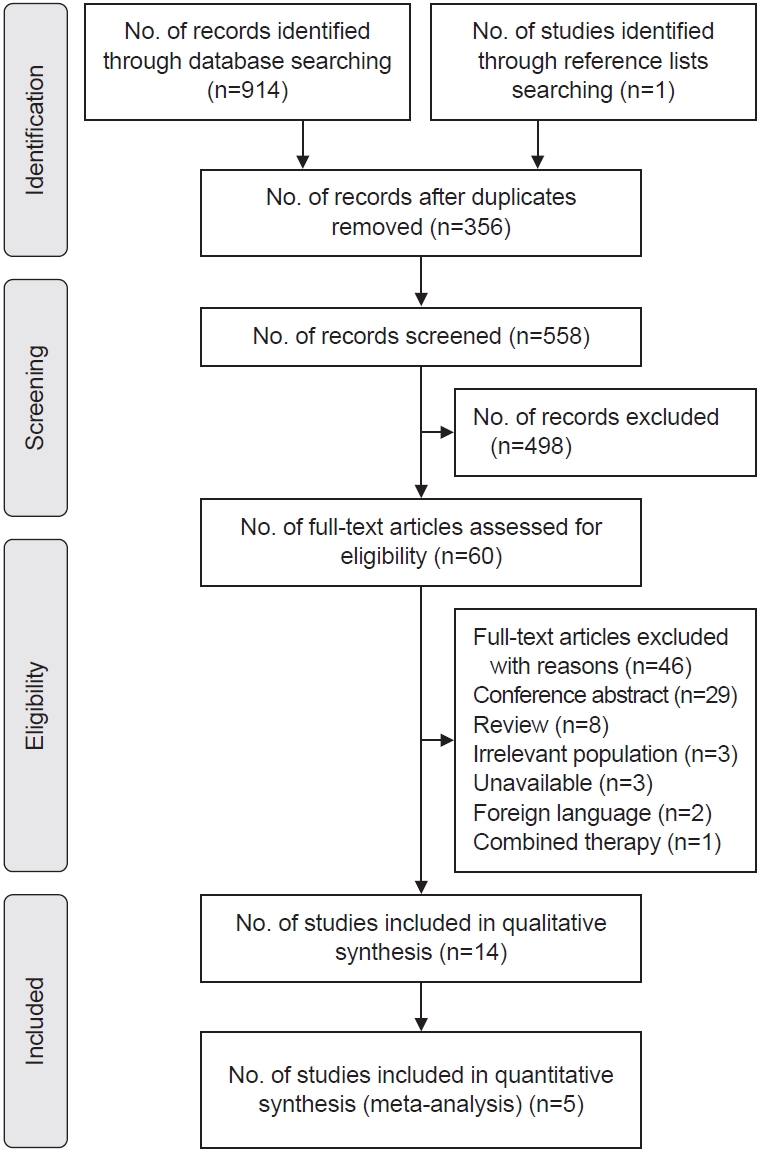
Our screening process is presented in Fig. 1. Initially, 914 potentially relevant articles were searched through the electronic databases, and one additional citation was identified by manual searching. After removing duplicates (n=356), 558 studies were screened in view of the title, abstract, or full text, and 498 were discarded. The remaining 60 studies were included for full-text evaluation. Finally, 14 articles met the eligibility criteria and included our review, and 46 studies were excluded with reasons (Supplementary Table S2).
Characteristics of included studies
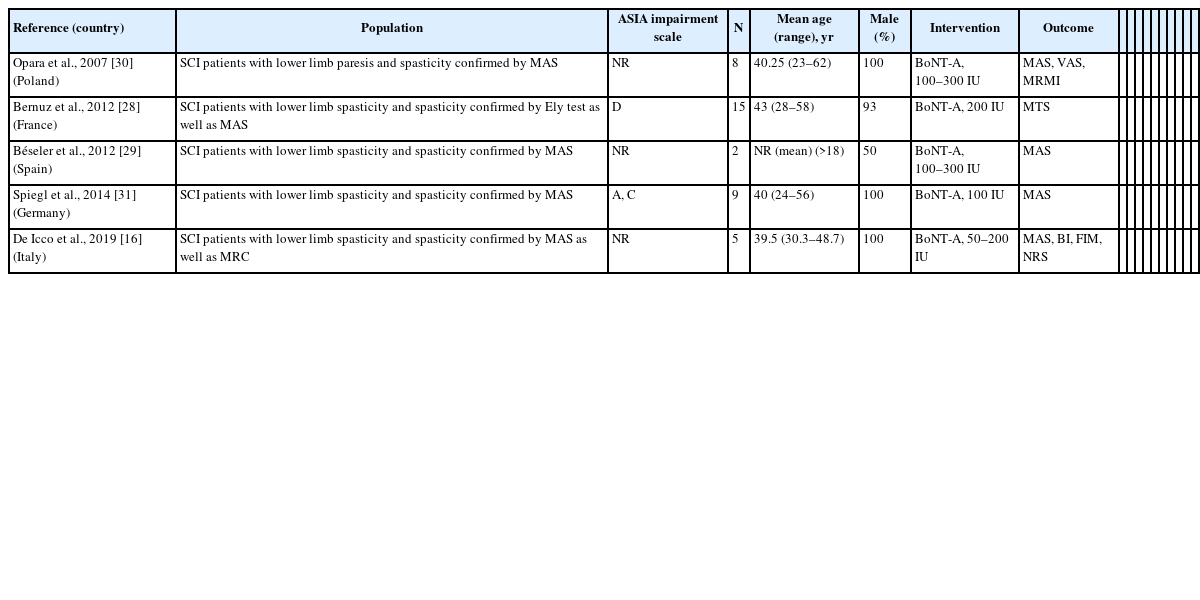
Fourteen studies investigated the effect of Botox-A for the management of spasticity in SCI patients. The citation search identified two RCTs [15,27], five pre-post designs [16,28-31], and seven case report studies [32-38]. An overview of assessments of outcomes measured in the intervention is shown in Table 1. The MAS and VAS were the most common outcome measures for spasticity and pain assessment respectively. Regarding activity of daily living, the BI was adopted most, followed by GAS and FIM evaluation. The gait analysis was frequently adopted for the locomotion activity. Only one RCT evaluated the quality-of-life outcome by DAS scoring. A detailed characteristic of the selected studies is shown in Tables 2-4.
Quality assessment
The methodological quality of the included studies was presented in Supplementary Table S3 (RCTs), Supplementary Table S4 (pre-post studies), and Supplementary Table S5 (case reports). According to the results of quality of evidence, all RCTs had appraised of high quality [15,27], three out of five pre-post trials had appraised of good quality [19,29,30], and two studies rated as fair quality [28,31]; and three out of seven case report studies had appraised of high quality [32,36,37], and rest of four were moderate quality [33-35,38].
Meta-analysis
Effects of Botox-A in pre-post studies on the spasticity
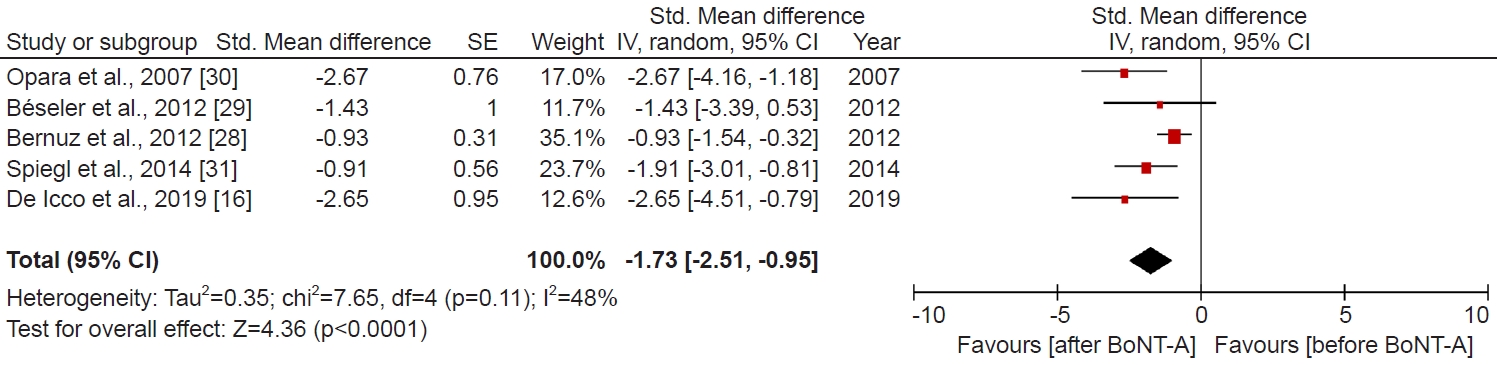
Short-term (2–4 weeks) Botox-A treatment was effective as determined by the MAS and MTS scales. Taken together, four studies (24 patients) that used MAS and one study (15 patients) MTS to measure spasticity showed that short-term Botox-A treatment significantly decreased spasticity (SMD, -1.73; 95% CI, -2.51 to -0.95; p<0.0001, I2=48%; Fig. 2). We imputed the 0.5 correlation coefficients to ascertain missing SDs. To reduce bias, we performed the sensitivity analysis and applied correlation coefficients of 0.8 and 0.0. Using a correlation coefficient of 0.8 (SMD, -1.86; 95% CI, -2.64 to -1.07; p<0.00001, I2=80%; Supplementary Fig. S1), as well as 0.0 (SMD, -1.45; 95% CI, -2.11 to -0.78; p<0.0001, I2=1%; Supplementary Fig. S2), the pooled difference between before Botox-A treatment and after Botox-A treatment remained statistically significant.
Effects of Botox-A in pre-post studies on the pain
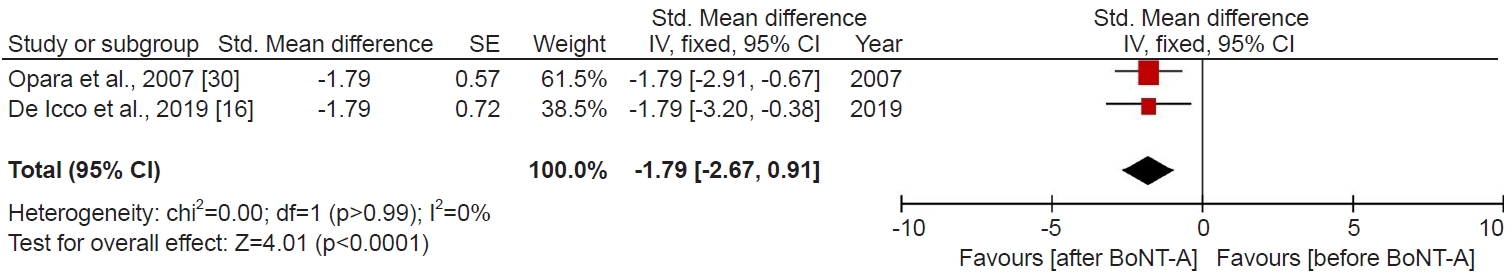
Short-term (3–4 weeks) Botox-A treatment significantly reduced the pain as determined using the VAS and NRS scales. Altogether, one study (8 patients) that used VAS and one study (5 patients) NRS to measure pain showed that short-term Botox-A treatment significantly decreased pain (SMD, -1.79; 95% CI, -2.67 to -0.91; p<0.0001, I2=0%; Fig. 3). We imputed the 0.5 correlation coefficients to ascertain missing SDs. As the sensitivity analysis, we applied correlation coefficients of 0.8 and 0.0. Using a correlation coefficient of 0.8 (SMD, -1.79; 95% CI, -2.34 to -1.24; p<0.00001, I2=0%; Supplementary Fig. S3), as well as 0.0 (SMD, -1.79; 95% CI, -3.01 to -0.57; p=0.004, I2=0%; Supplementary Fig. S4), the pooled difference between before Botox-A treatment and after Botox-A treatment remained statistically significant.
Systematic review
Effects of Botox-A on spasticity in RCTs
Two RCTs were included in our systematic review [15,27]. Both trials measured spasticity and found that Botox-A yielded a significant reduction of spasticity when compared with a comparator group. Yan et al. [15] compared the efficacy of Botox-A with baclofen and/or physical therapy at 2, 4, and 6 weeks of follow-up. Compared with the baseline score, at 2 weeks, physical therapies had no effect (p=0.063), baclofen (p=0.003), and Botox-A (p=0.0224) had decreased MAS scores. At 4 weeks, physical therapy (p<0.0001) baclofen (p<0.0001), and Botox-A (p<0.0001) had reduced MAS scores. Final follow-up after 6 weeks, baclofen (p=0.02) had not improved, and Botox-A (p=0.02) had decreased MAS score compared to physical therapy. In another study, Richardson et al. [27] compared the efficacy of Botox-A with placebo at 3, 6, 9, and 12 weeks follow-up. Across both groups, AS was significantly decreased at 3 weeks relative to baseline (p<0.0001) but when compared with subsequent successive time contrasts such as 6 weeks vs. 3 weeks; 9 weeks vs. 6 weeks; 12 weeks vs. 9 weeks were insignificant (p>0.2, in every case).
Effects of Botox-A on motor function, ADL, and QoL in RCTs
Richardson et al. [27] also assessed motor function and ADL by RMA and GAS scale respectively, but there was no group difference in aggregate outcome scores. Yan et al. [15] measured ADL by BI score and demonstrated that physical therapy was required for at least 6 weeks for increasing the BI scores (p<0.0001). However, baclofen (p<0.0001) and Botox-A (p=0.0015) showed increased BI scores within 2 weeks. After 6 weeks, baclofen and Botox-A had the same effects on BI scores and consistently increased BI scores during the follow-up period. Besides, Yan et al. [15] also measured QoL by DAS score and showed that baclofen relatively improved DAS score than Botox-A at 4 weeks (p=0.0496) and 6 weeks (p<0.0001), as well.
Effects of Botox-A on spasticity and pain in case studies
The anti-spasticity effect of Botox on individuals with SCI was reported in seven case studies. The authors' conclusions and population characteristics from included case studies were presented in Tables 2-4. Among the seven case studies, five studies featured SCI patients with lower limb spasticity [32-36], while two studies included upper limb spasticity [37,38]. In addition, five case studies [32-36] also measured pain by different pain assessment scales, including VAS, and NRS. A study by Frost et al. [33] demonstrated that preoperative Botox-A reduces postoperative spasticity and pain, and eliminated the need for gait aids in patients with SCI-spasticity. Similarly, Botox-A treatment of spasticity in SCI patients reduced lower limb spasticity and spasticity-related pain after 24–72 hours [32]. Consistent with these findings, Al-Khodairy et al. [32] also reported that the clinical effects initiate within 24–72 hours after the Botox-A injection, peak in around 4–6 weeks, and are sustained for more than 3 months. The results, as shown by Htwe et al. [35], indicated that Botox-A response was very promising with a significant reduction in lower limb MAS score. Gross et al. [34] further disclosed that Botox-A markedly decreased spasticity of the rectus femoris. Naicker et al. [36] reported that Botox-A reversed the spasticity, spasticity-associated pain in the lower limb as indicated by SCI patient decreased MAS, VAS score, and sleep quality. In the upper limb, Botox-A treatment notably reduced AS (from 3 to 1) and maintain over 12 weeks [34]. Furthermore, the VAS score was significantly decreased by Botox-A injection, and the patient was able to shake hands during the 12-week study period [34]. Similarly, Tang et al. [38] found that Botox-A reduced spasticity and pain in upper limb muscles, including biceps, flexor digitorum superficilais, brachialis, and pronator teres muscles.
Certainty of the evidence
The certainty of evidence for the efficacy of Botox-A on spasticity and pain outcomes was assessed using the GRADE approach. Details of GRADE certainty of evidence for the spasticity outcomes are shown in Supplementary Table S6.
DISCUSSION
This systematic review of the literature summarized the available evidence on the effects of Botox-A for the management of spasticity in SCI patients. We identified two RCTs [15,27], five pre-post studies [18,28-31] and seven case reports [32-38], from three major electronic databases. The meta-analysis from pre-post studies suggested that patients with SCI could eventually show benefits regarding decreased spasticity (p<0.0001) within 2–4 weeks of Botox-A treatment. However, as depicted in Fig. 2, one study [29] approached the threshold of no effect, suggesting that Botox-A may not significantly improve spasticity outcomes. This study included two SCI patients: one female at 4 months post-onset and one male at 3 years post-onset [29], highlighting the potential impact of elapsed time between SCI onset and treatment administration on Botox-A effectiveness for spasticity. A further study is imperative to determine the optimal timing of Botox-A administration relative to SCI onset for achieving maximum efficacy in improving spasticity outcomes. Furthermore, Botox-A treatment may be also effective in SCI for the reduction of nociceptive pain (p<0.0001). Our quality assessment of included studies indicates the pre-post studies are fair to good quality. We rated all RCTs are high quality, and case reports are moderate to high quality. It is important to note that due to variation of the control group between two included RCTs, we did not perform the pairwise meta-analysis. In addition, case reports based on a single patient do not permit the estimation of effect size. Thus, the findings of RCTs and case reports were narratively described.
Despite our ample efforts, we were only able to include 14 studies in this review. Of these, two (RCTs) are level 1, five (pre-post) studies are level 4, and seven (case reports) are level 5 evidence [39]. The encouraging findings of our study that we are included two RCTs and performed for the first-time meta-analysis based on pre-post design studies in the SCI population. Consistent with other studies our meta-analysis has shown that Botox-A significantly reduced lower limb spasticity in SCI patients [40]. Recently, Yan et al. [15] performed one large RCTs to evaluate the efficacy of Botox-A in SCI patients. The results of this study also corroborated our findings that Botox-A notably decreased MAS score after a 2, 4, and 6 weeks follow-up period. Richardson et al. [37] compared the efficacy of Botox-A with the placebo up to 12 weeks follow-up period. This RCT only included 6 SCI patients (placebo group=2, and Botox-A group=4) and also concluded that Botox-A significantly decreased lower limb spasticity. In our systematic review, we only retrieved two RCTs and outcome measures estimated for short follow-up periods (2 to 12 weeks). In-future, long-term follow-up studies in RCT design are urgently required to establish the efficacy of Botox-A in spasticity caused by SCI. Our meta-analysis based on level 4 evidence in SCI showed very promising results for spasticity management through Botox-A, but meta-analysis based on level 1 evidence is required to confirm our findings before getting approval for this drug. In the future, a well-design, multicenter RCT on the current topic is therefore recommended. While we conducted searches across databases without restricting by language or publication date, we excluded non-English language articles and conference abstracts. This exclusion may introduce publication bias and result in an incomplete representation of the available evidence. Future studies should consider including non-English literature, grey literature like conference abstracts, and expanding search strategies to encompass country-specific databases such as CNKI and WANFANG, thus ensuring a comprehensive representation of available evidence.
Abnormal posture and soft tissue changes by spasticity can aggravate the risk of pain, in turn, pain may worsen spasticity[18]. Thus, patients with SCI-spasticity and spasticity-related pain can enter a vicious circle that can intensify both pain and spasticity. Most recently, a cross-sectional survey reported that spasticity and chronic pain commonly appears following SCI [41]. Tibbett et al. [1] also reported that spasticity and chronic pain are directly related, which significantly impact ADL in SCI patients. Our included case reports [32-38] also support the idea that spasticity and pain are interrelated. Interestingly, Botox-A treatment not only reduced the spasticity but also pain in SCI patients [27,32,33,35,36,38]. Our meta-analysis based on level 4 evidence demonstrated that Botox-A treatment markedly reduced nociceptive pain (p<0.0001) in the SCI population. Surprisingly, no RCTs yet evaluated the efficacy of Botox-A to reduce nociceptive pain in SCI subjects. However, our included RCTs reported other beneficial effects of Botox-A in SCI, including improvement of motor function, ADL, and QoL [15,27]. Further research should be undertaken to investigate the salutary effects of Botox-A for the management of spasticity and nociceptive pain in SCI are therefore recommended.
CONCLUSION
This systematic review and meta-analysis provide promising insights into the potential of Botox-A therapy in alleviating spasticity and nociceptive pain among individuals with SCI, supported by pre-post studies and case reports. Moving forward, there is a critical need for additional high-quality RCTs with extended follow-up periods to firmly establish the efficacy of Botox-A in managing SCI. Furthermore, our study underscores the complex relationship between spasticity and pain in SCI patients, suggesting the potential for Botox-A treatment to address both conditions simultaneously. Future research should explore the broader impact of Botox-A therapy on motor function, ADL, and overall QoL within this demographic, advancing our understanding of its clinical application in treating spinal cord injuries.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING INFORMATION
This work was supported in part by grants from the National Research Foundation (2017R1D1A1B0302956514, 2020R1A2C201215511 to Y.H.), Korea.
AUTHOR CONTRIBUTION
Conceptualization: Sumsuzzman DM, Hong Y. Methodology: Sumsuzzman DM, Khan ZA, Nila IS, Moran VMV. Formal analysis: Sumsuzzman DM, Khan ZA, Nila IS, Moran VMV. Funding acquisition: Hong Y. Project administration: Sumsuzzman DM, Hong Y. Visualization: Sumsuzzman DM, Rajesh M, Yang WJ. Writing – original draft: Sumsuzzman DM, Hong Y. Writing – review and editing: Sumsuzzman DM, Khan ZA, Nila IS, Moran VMV, Rajesh M, Yang WJ, Hong Y. Approval of final manuscript: all authors.
Acknowledgements
The authors would like to acknowledge the invaluable support and critical comments of members in the ‘Biological Clock and Aging Control’ laboratory. This study was completed as part of the doctoral dissertation by D.M.S.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5535/arm.240034.
Supplementary Material
PRISMA 2020 Checklist
Supplementary Table S1.
Electronic databases search strategy
Supplementary Table S2.
Exclusion criteria
Supplementary Table S3.
Methodological quality assessment of randomized controlled studies
Supplementary Table S4.
Methodological quality assessment of pre-post studies
Supplementary Table S5.
Methodological quality assessment of case-report studies
Supplementary Fig. S1.
When correlation coefficient=0.80. Forest plot comparing the changes in the spasticity score between before botulinum toxin-A (BoNT-A) and after BoNT-A treatment in spinal cord injury. 95% CI, 95% confidence interval; SE, standard error; IV, independent variable.
Supplementary Fig. S2.
When correlation coefficient=0.00. Forest plot comparing the changes in the spasticity score between before botulinum toxin-A (BoNT-A) and after BoNT-A treatment in spinal cord injury. 95% CI, 95% confidence interval; SE, standard error; IV, independent variable.
Supplementary Fig. S3.
When correlation coefficient=0.80. Forest plot comparing the changes in the pain score between before botulinum toxin-A (BoNT-A) and after BoNT-A treatment in spinal cord injury. 95% CI, 95% confidence interval; SE, standard error; IV, independent variable.
Supplementary Fig. S4.
When correlation coefficient=0.00. Forest plot comparing the changes in the pain score between before botulinum toxin-A (BoNT-A) and after BoNT-A treatment in spinal cord injury. 95% CI, 95% confidence interval; SE, standard error; IV, independent variable.
Supplementary Table S6.
GRADE summary of findings table