- Search
| Ann Rehabil Med > Volume 46(5); 2022 > Article |
|
Abstract
Objective
To investigate esophageal motility disorders in patients with esophageal residual barium on chest x-rays after videofluoroscopic swallowing studies (VFSS) through high-resolution esophageal manometry (HREM).
Methods
We reviewed the records of 432 patients who underwent VFSS from September 2019 to May 2021, and 85 patients (19.7%) with large residual barium (diameter ≥1 cm) were included. As a result of HREM, motility disorders were classified as major or minor motility disorders according. Esophagogastroduodenoscopy and chest computed tomography results available were also reviewed.
Results
Among 85 patients with large residual barium in the esophagus, 16 patients (18.8%) underwent HREM. Abnormal esophageal motilities were identified in 68.8% patient: three patients (18.8%) had major motility disorders—achalasia (n=1), esophagogastric junction (EGJ) outflow obstruction (n=2)—and eight patients (50%) had minor motility disorders—ineffective esophageal motility (n=7), fragmented peristalsis (n=1). In those with normal esophageal motility, three patients of esophageal structure disorders (18.8%)—esophageal cancer (n=1), cardiogenic dysphagia (n=1), slight narrowing without obstruction of EGJ (n=1)—and two patients (12.5%) with chronic atrophic gastritis (n=2) were confirmed.
Conclusion
Esophageal motility disorders were identified in 68.8% of 16 patients with large esophageal residual barium with three patients in the major and eight patients in the minor categories. Residual barium in the esophagus was not rare and can be a sign of significant esophageal motility disorders.
Coexistence of oropharyngeal dysphagia and esophageal dysphagia have been reported in many cases [1]. Although the patient’s subjective complaint is the first important clue in differentiating between oropharyngeal dysphagia and esophageal dysphagia [2,3], many patients with esophageal dysphagia initially complained of pharyngeal symptoms [4,5]. In patients suspected of both esophageal and pharyngeal dysphagia, pharyngeal swallowing test is recommended first, primarily to differentiate the intra-airway aspiration [2]. Videofluoroscopic swallowing studies (VFSS) are one of the standard tests to evaluate pharyngeal swallowing function. We previously reported 7.5% of the patients undergoing the test showed some degree of esophageal dysphagia [6]. When barium in the esophagus with a diameter of 2 cm or more was observed after the VFSS test, lung cancer and esophageal cancer were confirmed through esophagogastroduodenoscopy (EGD) or chest computed tomography (CT) scan, and achalasia was confirmed through barium esophagogram [6].
Esophageal motility disorders are classified into major and minor motility disorders according to the Chicago Classification, based on findings of high-resolution esophageal manometry (HREM) [7]. No previous attempt has been made to classify patients with barium residues in the esophagus after VFSS through HREM. According to previous studies of timed barium esophagogram, an esophageal diameter 4 cm after 1 minute form barium swallowing suggested the possibility of achalasia, whereas a barium diameter greater than 1 cm suggested nonachalasia motility disorder [8,9]. We hypothesized that there would be esophageal motility disorders in patients with post-VFSS barium residues greater than 1 cm. In addition, we hypothesized that a significant number of patients undergoing the VFSS test may have difficulties in receiving the HREM due to pre-existing cognitive or physical impairments [6]. In this study, we aimed to confirm the presence of esophageal motility disorders by reviewing the results of HREM in subjects with a diameter of 1 cm or more of barium residue in the esophagus, and to determine the proportion of major motility disorders requiring active treatment attempts. We also aimed to analyze the proportion of those who did not receive the HREM and the reasons of HREM failures.
We retrospectively reviewed the records of 432 patients (aged ≥18 years; mean age 74.7±12.12 years) who underwent VFSS from September 2019 to May 2021 at Department of Rehabilitation Medicine, Kangwon National University Hospital. Post-VFSS chest X-ray were reviewed, 85 patients (19.7%) with large residual barium (diameter ≥1 cm) were included in the analysis. The Korean version of the Mini-Mental State Examination (MMSE), the Korean version of the Modified Barthel Index (MBI), and referral disorders (e.g., stroke) were reviewed. In patients who underwent HREM, the results of HREM and EGD or chest CT were reviewed (Fig. 1). When patients did not perform the HREM, the reasons for non-performance were reviewed, such as poor cooperation, refusal, or loss to follow-up. The study protocol was approved by the Institutional Review Board of the Kangwon National University Hospital (IRB No. 2021-06-022). This study was retrospective study and the informed consents from the patients were waived.
VFSS was tested using fluoroscopy (Luminos dRF MAX; Siemens, Munchen, Germany) with test foods mixed with barium contrast medium (Baritop HD barium powder; Kaigen Pharram Company Limited, Osaka, Japan) in predetermined order: thin fluid (2 mL, 5 mL), curd-type yogurt, pudding, rice gruel, rice, and thickened fluid. If there was significant endotracheal aspiration, the test was discontinued. The penetration-aspiration scale (PAS) was graded for each test food, ranging from 1 (no aspiration/penetration) to 8 (aspiration without coughing). The highest PAS value of all foods swallowed in the VFSS were reviewed; PAS grade 1 was classified as no aspiration/penetration, grade 2 to 5 as penetration, and grade 6 to 8 as aspiration [10]. According to the degree of dysphagia, four types of fluid thickening were recommended: thin (no thickening), nectar-thick, honey-thick, and pudding-thick [11]. When a thickened diet was not recommended due to the risk of aspiration, nasogastric tube feeding was recommended.
The chest X-ray image was obtained with digital X-ray equipments (Radnext 80, Hitachi, Tokyo, Japan; DigitalDiagnost 4 High Performance, Philips, Amsterdam, Nederland) following the VFSS examination. The interval from VFSS to chest X-ray was not strictly controlled, but was 5 to 10 minutes in most cases. The chest X-ray was reviewed to ascertain whether there was esophageal barium residue. To evaluate the severity, the diameter of residual barium in the esophagus was measured transversely at the widest point. For vertical localization, the end-level of esophageal residual barium was measured as the distance from the upper margin of the T1 vertebra (Fig. 2).
The HREM (Solar GI HRM; Laborie, Portsmouth, New Hampshire, USA) was recommended for evaluation of esophageal motility disorder. Motility disorders were classified according to the Chicago Classification version 3.0 [7]. Achalasia, esophagogastric junction (EGJ) outflow obstruction, diffuse esophageal spasm, jackhammer esophagus, or absent contractility were classified as major motility disorder, and other motility disorders were classified as minor motility disorder [7].
The HREM was performed according to the results of the swallowing function test. For those with no risk of aspiration, a conventional HREM protocol was used: swallow a thin liquid [7]. When oropharyngeal dysphagia was confirmed on VFSS and fluid thickening was recommended, the HREM protocol was modified to reduce the risk of aspiration during HREM: swallow a liquid mixed with a thickener (NUCARE Toromi Perpect; Daesang Life Science Corporation, Seoul, South Korea), as recommended by the VFSS. When nasogastric tube feeding was recommended as a result of the VFSS, HREM was not performed.
The HREM was performed in the supine position [7,12]. The examinee was asked to swallow 10 consecutive swallows of 5 mL of thin or thickened liquid at 30 second intervals. During the 30 second interval, patients were not allowed to swallow any saliva [12]. Medications, e.g., prokinetics, opioids, calcium channel blockers, that could affect esophageal motility were discontinued at least 24 hours prior to the test.
The independent t-test, chi-square test, Fisher exact test, and Mann-Whitney U test were used to compare those who succeeded with HREM with those who failed. The diameters and end-levels of esophageal barium residue between major motility disorders and minor motility disorders were analyzed using the Mann-Whitney U test. Statistical analyses were conducted using Statistical Package for Social Science, version 25.0 (IBM SPSS Statistics; IBM Corporation, Armonk, NY, USA). Statistical significance was defined as a p-value <0.05.
Age, sex, and referral disorders were reviewed for all 85 patients. MMSE and MBI were obtained in 69 of 85 patients. The mean age of the 85 patients was 79.3 years, and 48 (56.5%) were male. The most common referral cause was stroke (n=49; 57.6%). As a result of VFSS, penetration and aspiration were confirmed in 46 (54.1%) and 33 (38.8%), respectively (Table 1).
Of 85 patients, 69 patients (81.2%) did not undergo evaluation of esophageal motility disorders (failed HREM). Poor cooperation (n=17) was the most common reason for test failure followed by refusal or no visit (n=16). The reason for failed HREM was undefined in the medical charts of 27 patients. Other minor reasons were severe oropharyngeal dysphagia (n=1), poor general condition (n=5), and other medical conditions (n=2). One patient was referred to another hospital with a gastroenterologist, where the patient was previously treated (Fig. 1).
There was a significantly higher proportion of men in failed HREM workups compared to successful HREM (62.3% and 31.3%, respectively; p=0.024). However, there was no significant difference in age (p=0.300), referral disorders (p=0.430), VFSS findings (p=0.151), MBI (p=0.293), and MMSE (p=0.215) between those with failed HREM for all reasons and those with successful HREM (Table 1).
Of 85 patients with large esophageal residual barium, 16 (18.8%) successfully underwent both HREM (Fig. 1). Ten of the 16 patients underwent HREM with a thickened liquid according to the VFSS results (6, thin liquid; 8, nectar-thick; 2, pudding-thick) (Table 2).
As a result of HREM, esophageal motility disorder was identified in 11 (68.8%) out of 16 patients. Three patients (18.8%) had major motility disorders: achalasia (n=1) (Fig. 3) and EGJ outflow obstruction (n=2) (Fig. 4). Fig. 3 shows the HREM results of achalasia (case no#1) with integrated relaxation pressure >15 mmHg, 100% failed peristalsis, and panesophageal pressurization, which were reasonable findings for type II achalasia. For the treatment of esophageal dysphagia due to achalasia, a balloon catheter was dilatated at the EGJ. Fig. 4 shows integrated relaxation pressure > 15 mmHg and peristalsis in HREM (case no#2 and #3), which are compatible with for EGJ outflow obstruction. During and after meals, patients were instructed to maintain an upright posture for about 30 minutes. If necessary, prokinetic agents, such as serotonin modulating agents, were prescribed. Since no severe symptoms were identified, additional interventions (e.g., botulinum toxin injection, pneumatic dilation, esophagomyotomy) were not performed.
Eight patients (50%) had minor motility disorders: ineffective esophageal motility (n=7), fragmented peristalsis (n=1). Patients with minor motility disorder were instructed to eat in the upright position and to sit for about 30 minutes after eating.
In five patients with normal esophageal motility, three patients (18.8%) had esophageal structural disorders: esophageal cancer (n=1), cardiogenic dysphagia (n=1), slight narrowing without obstruction of EGJ (n=1). Although both HREM and EGD were performed, two patients (12.5%) had chronic atrophic gastritis (n=2) without significant abnormality in the esophagus (Fig. 1, Table 2).
Fig. 5 shows the EGD and chest CT images of an 81-year-old man who had the diameter of barium residue was 1.1 cm (case no#12), and the end-level of esophageal residual barium (distance from the upper margin of T1 body) was 13 cm on chest X-ray. On EGD and chest-CT, a cancer mass narrowing the lumen was observed at the same esophageal level as the barium residue, which corresponded to the chest X-ray. To palliate esophageal obstruction caused by cancer, stent insertion was performed through EGD (Fig. 5).
In a case of cardiogenic dysphagia, a rare cause of esophageal dysphagia [17], EGD showed external compression lesion in the mid-esophagus. On chest CT, the enlarged left atrium was observed to externally compress the midesophagus. After diuretic treatment, no barium residue was observed on post-VFSS chest X-rays followed up.
The diameter and end-level of residual esophageal barium were compared between major motility disorders and minor motility disorders. There was no significant difference in esophageal barium diameter between major motility disorders (median 1.8 cm, interquartile range [IQR] 1.3–2.5) and minor motility disorders (median 1.3 cm, IQR 1.1–1.5) (p=0.776). The end-levels of esophageal barium also showed no significant difference between major motility disorders (median 23.0 cm, IQR 19.2–24.0) and minor motility disorders (median 22.3 cm, IQR 17.4–27.0) (p=0.133) (Table 3).
Esophageal evaluations including HREM were performed to assess the esophageal motility disorders. The proportion of esophageal motility disorders was 68.8%. Major and minor esophageal motility disorders were 18.8% and 50%, respectively. This was the first study to analyze the proportion of esophageal motility disorders through HREM in patients with esophageal barium residues with diameter greater than 1 cm on the post-VFSS chest X-ray. Of the 85 patients with esophageal barium residues, only 16 underwent HREM testing, and the remaining 81% did not achieve HREM testing. Characteristics such as age, causative disease, MMSE, MBI, and VFSS findings excluding sex, were not significantly different between successful and failed HREMs. In spite of low execution rate of HREM, our findings suggest that esophageal motility disorders may be present in a high proportion in patients with large residual barium on post-VFSS chest X-rays.
In this analysis, the proportion of subjects with esophageal barium greater than 1 cm was 19% of the subjects, which was even higher than the previously reported 7.5% with presence of esophageal barium residue regardless of the size of diameter [6]. The previous study showed significant differences in the age of subjects based on the presence or absence of esophageal barium residues [6]. The average age of the study population in the previous study was 73.5 years, and the age of population in this study was slightly older (mean age was 74.7 years). The proportion of esophageal abnormalities may vary depending on the characteristics of the patients (e.g., age) participating in the VFSS. Therefore, it suggests that the rate of esophageal dysphagia among people receiving VFSS test may vary by hospitals or regions. Nevertheless, we were able to suggest that a non-negligible proportion of VFSS patients could have esophageal dysphagia.
In spite of the high proportion of identified causes of esophageal dysphagia (87.5%) including 11 motility and three structural disorders, two patients showed unspecified findings on HREM and EGD. In the current study, VFSS was performed in an upright posture and HREM was performed supine. Sweis et al. [18] reported that in the upright position, the pressure of the EGJ could change due to an increase in hydrostatic pressure or anatomical change. When upright position swallows were included in the HREM, the results could be different to a supine test [19]. Generally, esophageal motility disorders are associated with both solid foods and liquids [3]. HREM with solid food had different findings than that with liquid food. Sweis et al. [18] reported that the integrated relaxation pressure was larger in solid swallows than in liquid ones because of increased friction between the bolus and the luminal wall. Our HREM protocol was to swallow liquid (thin or thick). When solid food swallowing or upright position swallow were included in the HREM, the results could differ a from liquid and supine test [19]. The Chicago classification, which classified esophageal motility disorders through HREM, was published in version 3.0 in 2015 and updated to version 4.0 in 2020. In the Chicago classification version 4.0, positional change (supine and upright position) and provocative testing (solid food) were included [20]. Because the Chicago classification version 4.0 provides detailed diagnostic threshold values for upright position and solid food swallowing, more accurate diagnosis will be possible if HREM is based on the Chicago classification version 4.0. At the time of our study, the Chicago classification version 3.0 was the latest version, so the upright position test or the solid food test could not be performed.
Previous study suggested the larger diameter barium in the esophagus could be associated with achalasia compared to non-achalasia motility disorders [8,9]. In our study, the diameter and end-level of esophageal residual barium were not significantly related to the severity of esophageal motility disorders. First, this may be because only large barium residues (diameter ≥1 cm) were included in the analysis. Second, too few patients were included, and it may have failed to achieve statistical significance due to the small sample. Third, this may be due to the failure to control the interval between VFSS and chest X-ray. The interval between two tests may increase, depending on several factors. When large amounts of pharyngeal residues were observed during VFSS, oropharyngeal suction was performed during or after VFSS. Depending on the patient’s walking ability, the travel time to the chest X-ray room after VFSS could be delayed.
Sixty-nine of 85 patients (81%) failed HREM. Poor cooperation was the most common cause of failed HREM. The conventional HREM protocol (based on Chicago classification version 3.0), which requires 10 swallows of 5 mL of liquid in the supine position [7], was difficult to perform in patients who did not cooperate. Shinjo et al. [21] reported that swallowing was subjectively more difficult in the supine position than in the upright position. Xiao et al. [19] reported that the diagnostic results were significantly consistent in the supine and upright positions. Therefore, for patients with risk of aspiration and poor cooperation, carefully performing only five swallows in the upright position can be considered.
This study has several limitations. First, it was a retrospective review, and additional workups were not performed in the absence of esophageal barium residue or with small residue. Therefore, a randomized controlled trial is needed to determine whether there is a statistically significant difference in the diagnostic rate of esophageal dysphagia by performing additional workups in a control group without esophageal barium residue. Second, of 85 patients with large esophageal barium residue, only 16 patients underwent esophageal evaluation. The sample was too small to evaluate the relationship between the degree of barium diameter and the severity of esophageal motility disorders. Third, chest X-ray posture was not consistent. Patients (n=25) who were able to stand were examined with the chest posteroanterior (PA) to observe a wider lung field. However, the patient (n=60) with difficulty in standing was examined in anteroposterior (AP) method because there was a risk of falling. We could not find any papers reporting the difference in esophageal diameter between chest PA and chest AP, but there may be a bias in measuring barium diameter.
In conclusion, of the 85 patients with large esophageal residual barium, 16 (18.8%) successfully underwent HREM. Lack of cooperation, systemic deterioration, and other medical reasons were the most common reasons limiting HREM. Esophageal motility disorders were identified in 68.8% of 16 patients with large esophageal residual barium with three patients in the major and eight patients in the minor categories. Including three patients (18.8%) with structural disorders, the proportion of identified causes of esophageal dysphagia was increased up to 87.5%. Since residual barium in the esophagus is not rare and can be a sign of significant esophageal disease, additional esophageal evaluation will be required.
AUTHOR CONTRIBUTION
Conceptualization: Baek S, Park J. Methodology: Baek S, Park J. Writing–original draft: Baek S, Park J. Writing–review and editing: Baek S, Park J, Nam SJ. Approval of final manuscript: all authors.
Fig. 1
Inclusion flow chart. VFSS, videofluoroscopic swallowing study; HREM, high-resolution esophageal manometry; EGJ, esophagogastric junction.
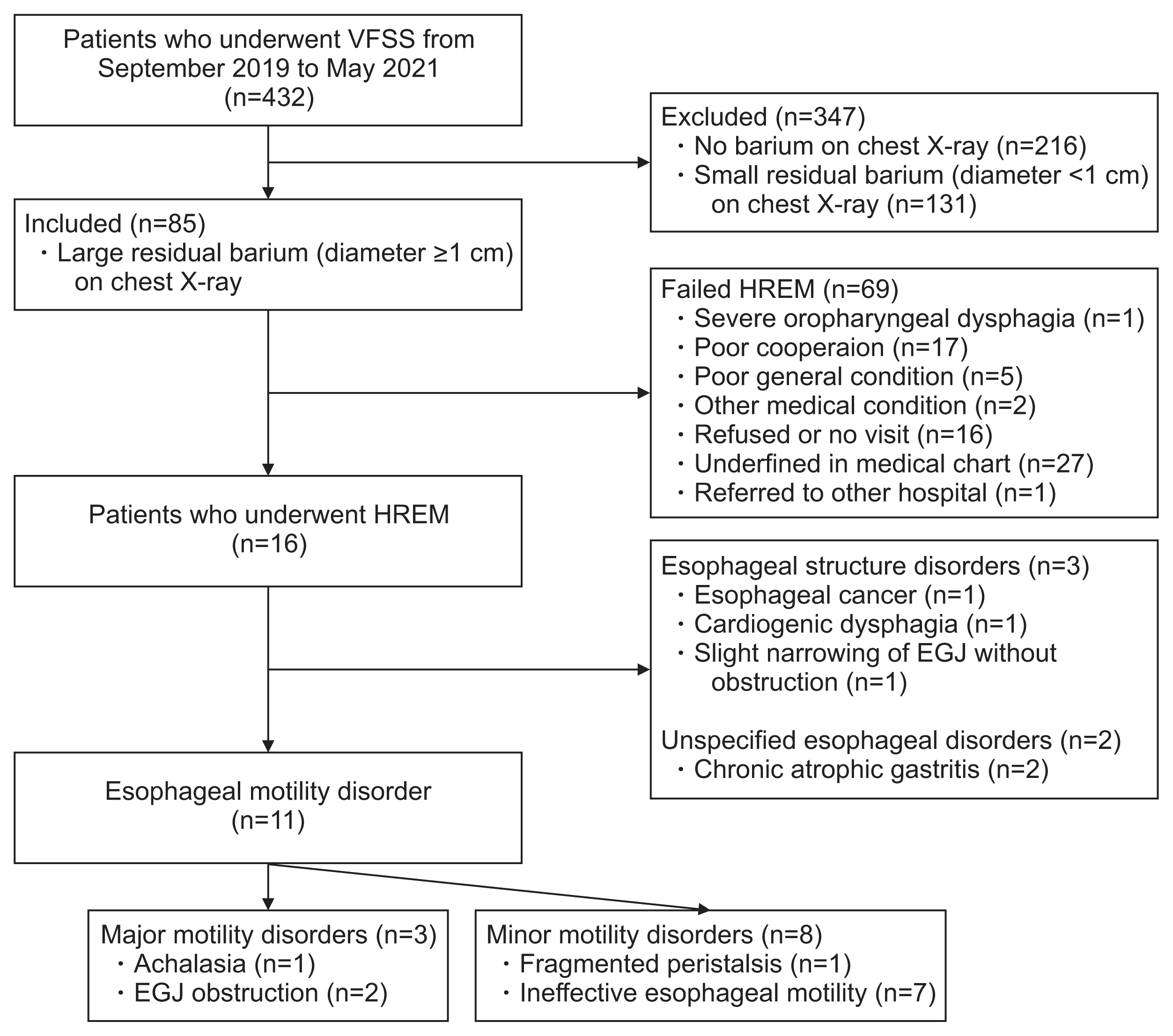
Fig. 2
Barium residues on chest X-rays taken after VFSS. (A) Barium content in the stomach with no residual esophageal barium (no barium group). (B) Small residual barium in the esophagus (<1 cm). End-level of esophageal barium was located 18.2 cm from T1 upper margin. (C) Large residual barium in the esophagus (barium ≥1 cm). End-level of esophageal barium was located 24.0 cm from T1 upper margin.

Fig. 3
Achalasia (case no#1). (A) Residual barium with a diameter of 2.5 cm was observed in the esophagus on chest X-ray. The end-level of the esophageal barium was located 23.0 cm from the T1 upper margin. Due to the fixed instrument, the bird beak sign is difficult to identify. (B) On esophageal manometry, impaired lower esophageal sphincter relaxation (integrated relaxation pressure >15 mmHg), absent peristalsis, and panesophageal pressurization were observed, which are reasonable findings for type II achalasia. (C) For the treatment of esophageal dysphagia due to achalasia, a balloon-catheter dilatation was performed at the esophagogastric junction.
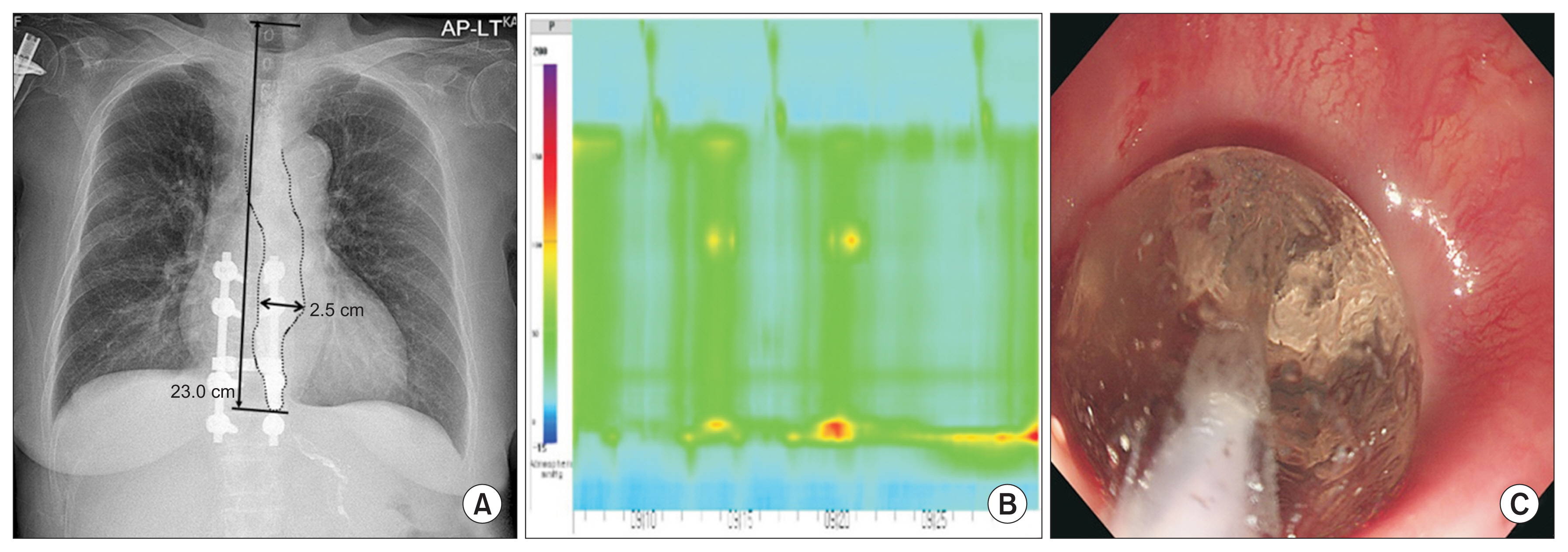
Fig. 4
Esophagogastric junction (EGJ) outflow obstruction (case no#2). (A) Residual barium with a diameter of 1.8 cm was observed in the esophagus on chest X-ray. The end-level of esophageal barium was located 24.0 cm from the T1 upper margin. (B) On esophageal manometry, impaired lower esophageal sphincter relaxation (integrated relaxation pressure >15 mmHg) but peristalsis was observed, which are reasonable findings for EGJ outflow obstruction.
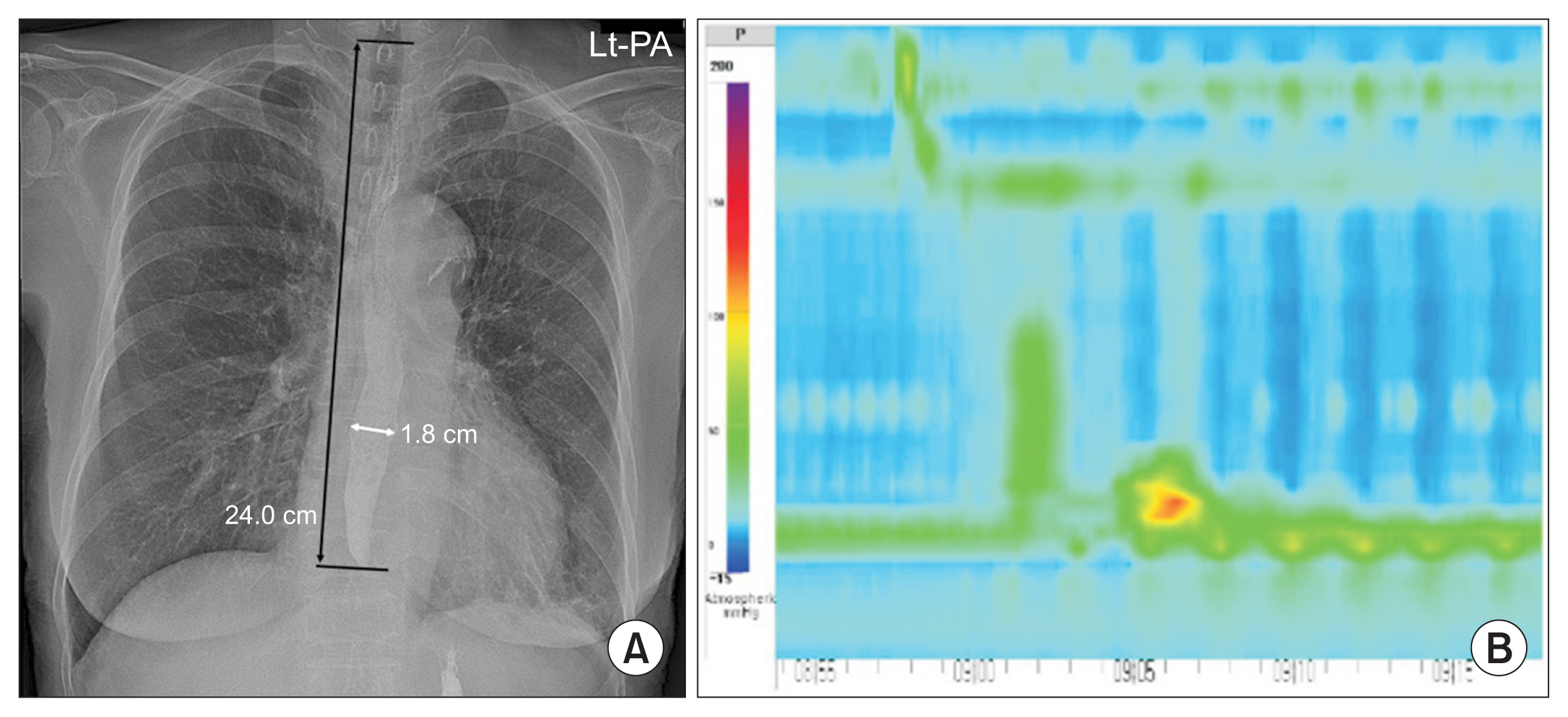
Fig. 5
Esophageal cancer (case no#12). (A) Residual barium with a diameter of 1.1 cm was observed in the esophagus on chest X-ray. The end-level of esophageal barium was located 13.0 cm from the T1 upper margin. A filling defect of the barium was observed for a length of 4.5 cm below the distal end of the barium, which was the middle thoracic esophageal level. (B) On esophagogastroduodenoscopy (EGD) and (C) chest computed tomography, a cancer narrowing the lumen was observed at the middle thoracic esophageal level, which corresponds to the chest X-ray. (D) To palliate esophageal obstruction caused by cancer, stent insertion was performed through EGD.

Table 1
Characteristics of patients with large residual barium and comparison of successful and failed HREM
| Variable | Barium diameter ≥ 1 cm (n=85) | Successful HREM (n=16) | Failed HREM (n=69) | p-value |
|---|---|---|---|---|
| Age (yr) | 79.3±10.31 | 76.9±9.98 | 79.9±10.38 | 0.300a) |
| Sex | 0.024*b) | |||
| Male | 48 (56.5) | 5 (31.3) | 43 (62.3) | |
| Female | 37 (43.5) | 11 (68.8) | 26 (37.7) | |
| Referral disorders | 0.430c) | |||
| Stroke | 49 (57.6) | 13 (81.3) | 36 (52.2) | |
| Traumatic brain injury | 4 (4.7) | 0 (0) | 4 (5.8) | |
| Parkinsonism | 5 (5.9) | 0 (0) | 5 (7.2) | |
| Dementia | 9 (10.6) | 1 (6.3) | 8 (11.6) | |
| Other brain lesion | 2 (2.4) | 0 (0) | 2 (2.9) | |
| Poor general condition | 8 (9.4) | 0 (0) | 8 (11.6) | |
| Others | 2 (2.4) | 1 (6.3) | 1 (1.4) | |
| Unknown | 6 (7.1) | 1 (6.3) | 5 (7.2) | |
| VFSS findings | 0.151c) | |||
| No aspiration or penetration | 6 (7.1) | 3 (18.8) | 3 (4.9) | |
| Penetration | 46 (54.1) | 8 (50.0) | 38 (55.1) | |
| Aspiration | 33 (38.8) | 5 (31.3) | 28 (40.6) | |
| MBI | 22 (8.0–54.5) (n=69) | 39 (12.0–64.0) (n=13) | 20 (8.0–49.5) (n=56) | 0.293d) |
| MMSE | 15.4±8.42 (n=69) | 12.77±9.36 (n=13) | 16.0±8.16 (n=56) | 0.215a) |
Table 2
Esophageal examination results in 16 patients who underwent esophageal workup
| No. | Age (yr) | Sex | Referral disorders | VFSS results | Esophageal barium | Result of esophageal evaluations | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Aspiration or penetration | Recommended liquid diet | Diameter (cm) | Endlevela) (cm) | Classification of disorders | HREM | Others | ||||
| 1 | 73 | F | Stroke | No aspiration or penetration | Thin | 2.5 | 23.0 | Major motility | Achalasia | Resistance of low esophageal sphincterb) |
|
|
||||||||||
| 2 | 82 | F | Stroke | Aspiration | Nectar-thick | 1.8 | 24.0 | Major motility | Esophagogastric junction outflow obstruction | Normalb) |
|
|
||||||||||
| 3 | 76 | F | Unknown | Penetration | Thin | 1.3 | 19.2 | Major motility | Esophagogastric junction outflow obstruction | Normalb) |
|
|
||||||||||
| 4 | 78 | F | Stroke | Penetration | Thin | 1.3 | 28.2 | Minor motility | Fragmented peristalsis | Normalb) |
|
|
||||||||||
| 5 | 56 | F | Stroke | No aspiration or penetration | Thin | 1.2 | 18.5 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 6 | 97 | F | Stroke | Aspiration | Nectar-thick | 1.1 | 17.0 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 7 | 77 | F | Stroke | Penetration | Nectar-thick | 1.2 | 24.0 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 8 | 85 | F | Stroke | Aspiration | Nectar-thick | 1.4 | 22.0 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 9 | 64 | M | Stroke | No aspiration or penetration | Thin | 1.1 | 22.5 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 10 | 75 | F | Stroke | Penetration | Nectar-thick | 1.5 | 14.0 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 11 | 90 | M | Dementia | Aspiration | Pudding-thick | 2.1 | 28.0 | Minor motility | Ineffective esophageal motility | Normalb) |
|
|
||||||||||
| 12 | 81 | M | Anterior cervical osteophyte | Aspiration | Nectar-thick | 1.1 | 13.0 | Structure disorder | Normal | Esophageal cancerb,c) |
|
|
||||||||||
| 13 | 74 | F | Stroke | Penetration | Pudding-thick | 1.5 | 11.5 | Structure disorder | Normal | Cardiogenic dysphagiab,c) |
|
|
||||||||||
| 14 | 64 | M | Stroke | Penetration | Nectar-Thick | 1.0 | 27.5 | Structure disorder | Normal | Narrowing without obstruction of esophagogastric junctionb) |
|
|
||||||||||
| 15 | 80 | M | Stroke | Penetration | Nectar-thick | 1.8 | 21.7 | Unspecified | Normal | Chronic atrophic gastritisb) |
|
|
||||||||||
| 16 | 78 | F | Stroke | Penetration | Thin | 1.1 | 15.0 | Unspecified | Normal | Chronic atrophic gastritisb) |
Table 3
Association of esophageal barium diameter degree and distance between distal end of barium and upper margin of T1 in major motility disorders
| Esophageal barium | Major motility disorders (n=3) | Minor motility disorders (n=8) | p-valuea) |
|---|---|---|---|
| Diameter (cm) | 1.8 (1.3–2.5) | 1.3 (1.1–1.5) | 0.776 |
| Distance to T1 upper margin (cm) | 23.0 (19.2–24.0) | 22.3 (17.4–27.0) | 0.133 |
REFERENCES
1. Triadafilopoulos G, Hallstone A, Nelson-Abbott H, Bedinger K. Oropharyngeal and esophageal interrelationships in patients with nonobstructive dysphagia. Dig Dis Sci 1992;37:551-7.



2. Liu LW, Andrews CN, Armstrong D, Diamant N, Jaffer N, Lazarescu A, et al. Clinical practice guidelines for the assessment of uninvestigated esophageal dysphagia. J Can Assoc Gastroenterol 2018;1:5-19.



3. Cockeram AW. Canadian Association of Gastroenterology practice guidelines: evaluation of dysphagia. Can J Gastroenterol 1998;12:409-13.



4. Yoon SW, Kim KW, Park HJ, Kim EK, Yu JS, Seo JK, et al. The usefulness of esophagogram with marshmallow bolus in patients with esophageal-related symptoms. J Korean Radiol Soc 1996;34:399-404.

5. Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998;171:1361-5.


6. Min YK, Baek S, Kang EK, Nam SJ. Characteristics of patients with esophageal dysphagia assessed by chest x-ray imaging after videofluoroscopic swallowing study. Ann Rehabil Med 2020;44:38-47.




7. Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74.



8. Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol 2018;113:196-203.


9. Kostic S, Andersson M, Hellstrom M, Lonroth H, Lundell L. Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis Esophagus 2005;18:96-103.


10. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 1996;11:93-8.



11. Park J, Yoo W, Yoo B. Standard recipes for the preparation of thickened barium liquids used in the diagnosis of dysphagia. Clin Nutr Res 2019;8:265-71.




12. Richter JE, Wu WC, Johns DN, Blackwell JN, Nelson JL, Castell JA, et al. Esophageal manometry in 95 healthy adult volunteers: variability of pressures with age and frequency of “abnormal” contractions. Dig Dis Sci 1987;32:583-92.

13. Varadarajulu S, Eloubeidi MA, Patel RS, Mulcahy HE, Barkun A, Jowell P, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005;61:804-8.


14. Qureshi NA, Hallissey MT, Fielding JW. Outcome of index upper gastrointestinal endoscopy in patients presenting with dysphagia in a tertiary care hospital: a 10 years review. BMC Gastroenterol 2007;7:43.




17. Park J, Baek S, Kim G, Nam SJ, Cho BR. Dysphagia secondary to esophageal compression in a patient with decompensated heart failure. Korean J Helicobacter Up Gastrointest Res 2022;22:146-51.


18. Sweis R, Anggiansah A, Wong T, Kaufman E, Obrecht S, Fox M. Normative values and inter-observer agreement for liquid and solid bolus swallows in upright and supine positions as assessed by esophageal high-resolution manometry. Neurogastroenterol Motil 2011;23:509-e198.


19. Xiao Y, Nicodeme F, Kahrilas PJ, Roman S, Lin Z, Pandolfino JE. Optimizing the swallow protocol of clinical high-resolution esophageal manometry studies. Neurogastroenterol Motil 2012;24:e489-96.



20. Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0. Neurogastroenterol Motil 2021;33:e14058.


21. Shinjo Y, Okitsu A, Ukeda I, Miyagi A, Domen K, Koyama T. Effects of posture on subjective swallowing difficulty during screening tests for dysphagia. Int J Phys Med Rehabil 2013;1:133.
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 2,743 View
- 79 Download
- Related articles in ARM
-
The Safety of Videofluoroscopic Swallowing Study (VFSS).2000 April;24(2)






