Robot-Assisted Gait Training in Individuals With Spinal Cord Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Article information
Abstract
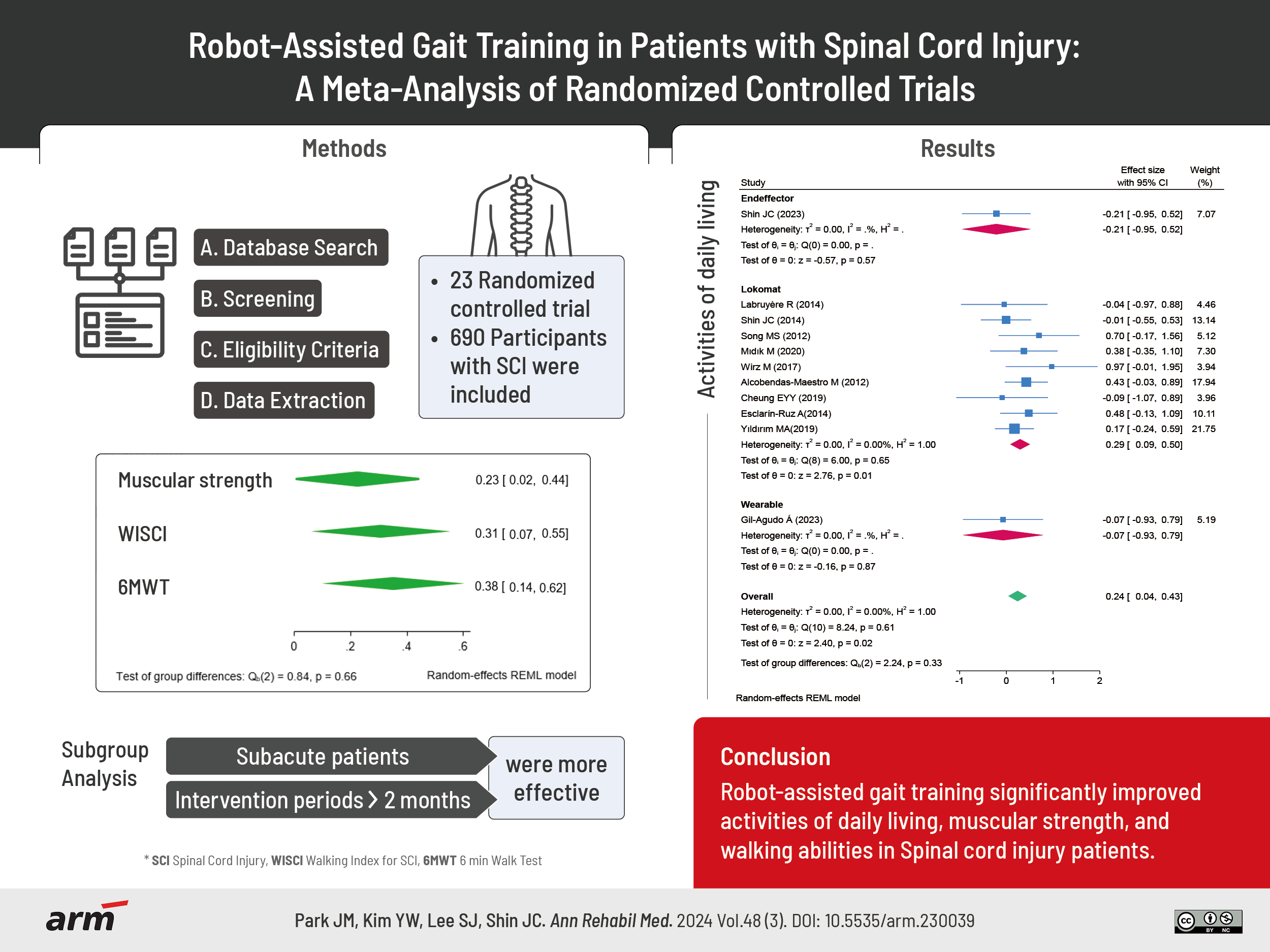
Spinal cord injury (SCI) rehabilitation emphasizes locomotion. Robotic-assisted gait training (RAGT) is widely used in clinical settings because of its benefits; however, its efficacy remains controversial. We conducted a systematic review and meta-analysis to investigate the efficacy of RAGT in patients with SCI. We searched international and domestic databases for articles published until April 18, 2024. The meta-analysis employed a random effects model to determine the effect size as either mean difference (MD) or standardized MD (SMD). Evidence quality was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Twenty-three studies with a total of 690 participants were included in the final analysis. The overall pooled effect size for improvement in activities of daily living was 0.24, with SMD (95% confidence interval [95% CI], 0.04–0.43; GRADE: high) favoring RAGT over conventional rehabilitation. Muscular strength (MD, 0.23; 95% CI, 0.02–0.44; GRADE: high), walking index for SCI (MD, 0.31; 95% CI, 0.07–0.55; GRADE: moderate) and 6 min walk test distance (MD, 0.38; 95% CI, 0.14–0.63; GRADE: moderate) showed significant improvement in the robot group. Subgroup analysis revealed that subacute patients and intervention periods >2 months were more effective. This meta-analysis revealed that RAGT significantly improved activities of daily living, muscular strength, and walking abilities. Additional studies are needed to identify the optimal treatment protocol and specific patient groups for which the protocol is most effective.
INTRODUCTION
Spinal cord injury (SCI) can cause various impairments in sensory, motor, and autonomic functions below the level of injury. This results in muscle weakness and atrophy, gait disturbances, sensory dysfunctions, and autonomic dysfunction [1]. Rehabilitation has focused on locomotion, especially for individuals with incomplete SCI classified as C or D on the American Spinal Injury Association (ASIA) Impairment Scale, because they are more likely to regain functional gait [2]. Early gait rehabilitation programs used manually assisted overground training with manual contact by a therapist, which later evolved into body weight-supported treadmill training. Since the introduction of the Lokomat (Hocoma AG) in the late 1990s, gait training with robots has gained traction, and is increasingly being used clinically because of its ability to perform high-intensity, repetitive movements for consistent periods of time, and is less demanding on the therapist [3].
With advancements in robotic technology, various types of robots are being utilized clinically, which can be broadly divided into three categories: exoskeleton treadmill training, wearable exoskeleton, and end-effector robots. Grounded exoskeleton robots have two programmable robotic joints in each leg, and are designed to support hip and knee movement when walking on a treadmill while supporting the body weight with a harness, with Lokomat being a prime example [3]. Wearable exoskeleton robots are ground walking systems that use a rigid external frame to support the lower limbs and torso while assisting movements at the hip and knee joints. They can be trained across a range of environments, including indoor and outdoor settings, as well as with obstacles and stairs. Examples include Ekso (Ekso Bionics), HANK (Gogoa Mobility), AIDER (Buffalo Robot Technology), and ReWalk (ReWalk Robotics) [4,5]. The end effector robot has a footplate attached with a walking trajectory only for the patient’s feet, allowing the knees and hips to move freely. Examples include Morning Walk (Curexo) and G-EO systems (Reha Technology) [6,7].
In 2021, the Korea Institute of Health and Medical Research analyzed the clinical utility of robotic-assisted gait training (RAGT) compared to that of conventional rehabilitation in patients with SCI. No statistically significant differences were observed in walking ability, muscle strength, functional performance, spasticity, balance, and quality of life [8]. As the number of relevant studies has grown, subsequent meta-analyses of the international literature have shown that RAGT significantly improves spasticity, walking ability, distance, speed, function, and peak oxygen consumption [9-11]. However, no studies have analyzed the effects of the type of robot, intervention duration, or onset period. We aimed to obtain data from randomized clinical trials conducted so far and dissect them based on robot classification, intervention duration, and onset period, with the aim of offering a comprehensive overview of the efficacy of RAGT in patients with SCI. We also hope that this study will support future policy decisions in the medical technology sector and contribute to adequate health insurance coverage.
METHODS
This meta-analysis adhered to the guidelines outlined in the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [12]. Supplementary Material S1 includes the PRISMA checklist. Additionally, the review protocol was registered and assigned the registration number CRD42023475357 in the International Prospective Register of Systematic Reviews.
Search strategy
The systematic search was conducted in six international (PubMed, Embase, Cochrane library, Scopus, Web of Science, and Google Scholar) and five domestic (KoreaMed, KMBase, KISS, RISS, and NDSL) databases until October 5, 2023, and we updated our search on April 18, 2024. The search formula was as follows: (Gait OR “Gait disorder” OR walking OR “Lower Extremity” OR leg or foot or ankle or tibia or fibula or femur or thigh or lower limb) AND (paresis OR paralysis or hemiplegia or paraplegia) AND (Robotics OR automation OR computer-assisted) AND (rehabilitation OR “Physical Therapy Modalities” OR “Exercise Therapy” OR physical therapy or physiotherapy or kinesitherapy or exercise) AND (“spinal cord injury” OR SCI) (Supplementary Material S2). We selected all published randomized controlled trials (RCTs) that compare RAGT with conventional rehabilitation methods. No data or language restrictions were applied. Two reviewers (JMP and JCS) independently reviewed titles and abstracts.
Study selection and data extraction
The study inclusion criteria adhered to the PICOS (Population, Intervention, Comparison, Outcomes, Study design) framework. The target population comprised patients with SCI and gait impairment. The intended intervention involved RAGT. For comparison, we established a control group of patients who received standard physical therapy with a therapist or non-robotic device therapy as traditional rehabilitation treatment. Designated outcomes comprised several primary outcomes encompassing activities of daily living (functional independence measure [FIM], spinal cord independence measurement [SCIM], and modified Barthel index [MBI]), muscular strength (lower extremity motor score, LEMS), spasticity (modified Ashworth scale, MAS), and balance (Berg balance scale, BBS), and walking ability (walking index for SCI [WISCI], 10 meter walk test [10MWT] speed, 6 min walk test [6MWT] distance, and step length). Secondary outcomes included body composition (body mass, lean tissue, and fat tissue) and cardiopulmonary function (forced expiratory volume in the first second [FEV1], forced vital capacity [FVC], heart rate, and peak cough flow [PCF]). The study design specifically included RCTs, excluding non-human studies, cohort studies, case reports, and studies that did not conform to the PICOS framework.
Two reviewers (JMP and YWK) independently extracted relevant data, including the name of the first author, year of publication, patient demographic data, mean age, sample size, type of intervention (robot type), intervention protocol, control protocol, and outcomes. Information extraction was facilitated by ImageJ software version 1.53 (ImageJ software; National Institutes of Health, https://imagej.nih.gov) in instances where the study estimates were solely presented graphically without numerical reporting.
Quality assessment and GRADE evaluation
The quality of studies was assessed using the Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB2), which identified potential bias risks. The RoB2 comprises five elements: the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Each element was appraised for low risk of bias, concerns of bias, high risk of bias, or lack of information [13]. Two reviewers (JMP and YWK) independently assessed the risk of bias, and disagreements regarding the quality assessment were resolved through discussion with a third reviewer (JCS).
The certainty of evidence in the included meta-analyses was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool, which comprises five domains: (1) risk of bias; (2) inconsistency; (3) indirectness; (4) imprecision; and (5) publication bias. Ratings for certainty of evidence were high, moderate, low, and very low [14].
Statistical analysis
In this meta-analysis, we conducted a statistical standardization of the effect sizes related to RAGT in patients with SCI. Initially, we calculated the mean and standard deviation (SD) of the change-from-baseline values for intervention and control groups. For studies lacking complete data, the mean and SD values of the change-from-baseline were computed, according to the guidelines outlined in Section 6 of the Cochrane Handbook (version 6.3). Effect sizes were calculated as mean difference (MD) and standardized MD (SMD), with 95% confidence interval (95% CI) within a random effects model. The included studies were from different countries and populations with different medical backgrounds; therefore, we conducted a restricted maximum likelihood random effects meta-analysis to account for an expected high level of heterogeneity [15]. Heterogeneity between studies was evaluated using the I2 metric for inconsistency and Cochrane Q test p-values. As suggested in previous studies [16,17] we assessed publication bias only in meta-analyses of ≥3 studies using funnel plots and Egger’s test. All statistical analyses were performed using Stata version 18 (StataCorp LP). Two-sided statistical tests were used, and significance was set at p<0.05. Subgroup analyses were performed according to robot type (treadmill training robot [Lokomat], wearable gait robot [Ekso, HANK, AIDER], end effector gait robot [morning walk]), intervention period, and onset period.
RESULTS
Study identification and characteristics
We screened 980 studies from international databases and 5,485 studies from domestic databases, excluding 102 and 334 duplicate records, respectively. After screening the titles and abstracts, 51 studies were selected from international and domestic databases, and their full texts were screened. Of these, 35 studies were excluded for various reasons (study protocol, observational study, review article, insufficient detail in the data, inappropriate intervention, and inappropriate control), and seven articles found through citation searches were combined, resulting in 23 studies that were finally included in the meta-analysis. Fig. 1 shows a flowchart outlining the study selection process. This meta-analysis included 690 patients with SCI: 346 and 344 in the intervention and control groups, respectively. The number of participants included in a single study ranged between 7 [18] and 88 [19]. The average age of the participants was between 8.4 [20] and 59.6 years [21]. The average onset duration of the participants was between 20 [22] and 7,665 days [23]. Overall characteristics of the included studies are summarized in Table 1 [18-40].

Screening and selecting studies using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart.
Assessment of risk and publication biases
Of the 23 RCTs, two showed high risk [24,25], but the rest showed some risk of concern. In all but one study [25], the randomization process and allocation were well described. Because the comparison was between RAGT and conventional standard physical treatment, all participants were aware of the intervention; therefore, bias due to deviations from the intended intervention was of some concern in all the studies. Approximately half of the studies (12 studies) showed a low risk of bias due to missing outcome data. In 11 studies (just under half of the included studies), outcome measure assessments were blinded to the intervention type, suggesting a low risk of outcome measure bias. Fourteen studies (more than half) pre-registered their study protocols and no data were duplicated, resulting in a low risk of bias in the selection of the reported results. Fig. 2 presents a traffic light diagram for each study included in the evaluation.

Traffic light diagram for the risk of bias of included studies as assessed using the Cochrane Risk of Bias 2.0 tool.
In Supplementary Fig. S1 illustrates the funnel plot, which displays the publication bias for each meta-analysis. We also checked for publication bias using the Eager test, as shown in Table 2. All analyses showed no significant publication bias, except for the step length and peak expiratory flow.
Effects of RAGT on activities of daily living
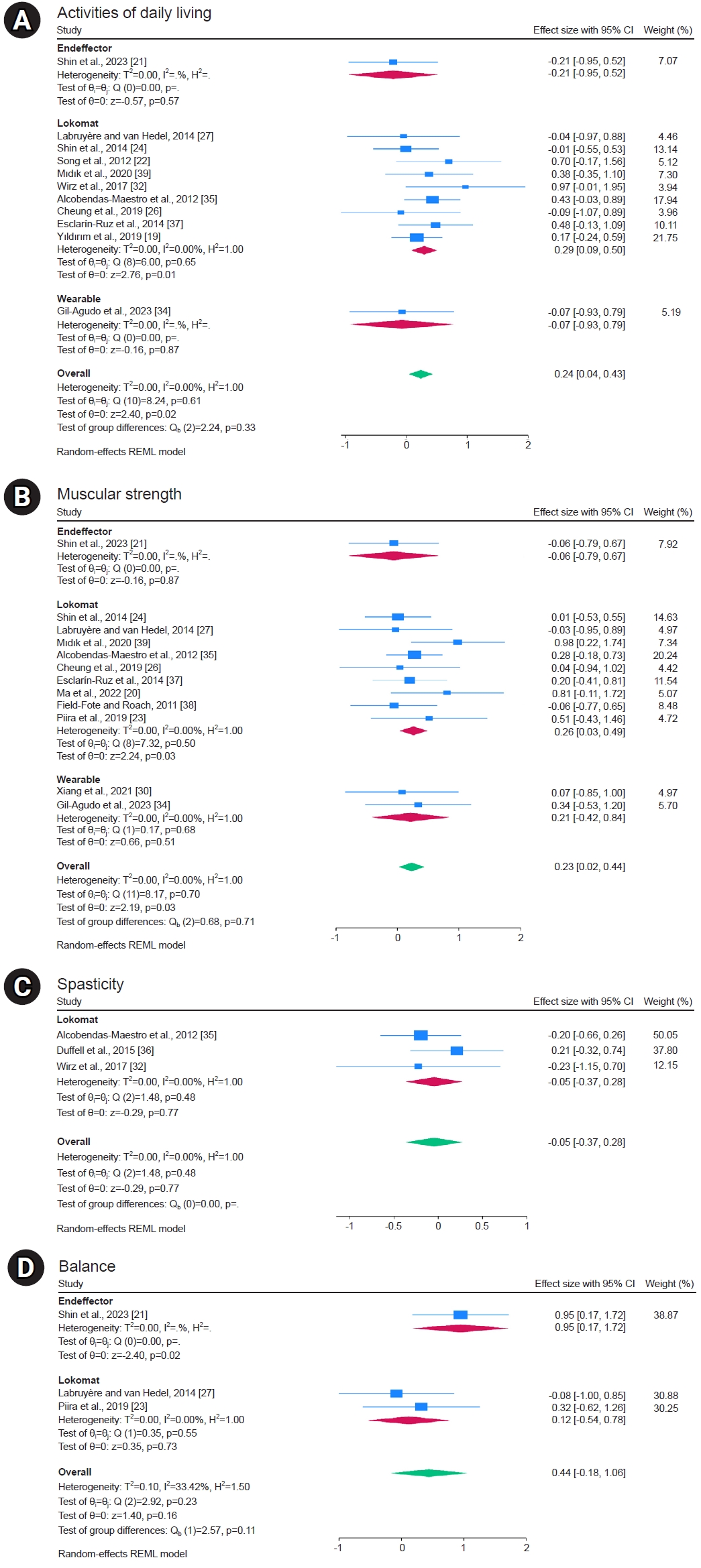
Activities of daily living, as measured using the FIM, SCIM, and MBI assessment tools, were reported in 11 studies. The overall meta-analysis showed significant improvement in the robot groups (SMD, 0.24; 95% CI, 0.04–0.43; I2=0%; p=0.02). When we analyzed the subgroups according to robot type, we found no significant difference in end-effector and wearable robots, but there was a statistically significant improvement in the Lokomat, a treadmill training robot (SMD, 0.29; 95% CI, 0.09–0.50; I2=0%; p=0.01; Fig. 3A). Activities of daily living quality of evidence was estimated to be high, according to the GRADE system (Table 2).
Effects of RAGT on muscular strength
Muscular strength measured using LEMS has been reported in 12 studies. The overall meta-analysis showed significant improvement in the robot groups (MD, 0.23; 95% CI, 0.02–0.44; I2=0%; p=0.03). Subgroup analysis according to robot type showed no significant difference in the end-effector and wearable robots, but a statistically significant improvement was observed in the Lokomat one (MD, 0.26; 95% CI, 0.03–0.49; I2=0%; p=0.03; Fig. 3B). The quality of evidence for muscular strength was estimated to be high using the GRADE system (Table 2).
Effects of RAGT on spasticity
Spasticity measured using the MAS was reported in three studies. The overall meta-analysis showed no significant difference (MD, -0.05; 95% CI, -0.37 to 0.28; I2=0%; p=0.77; Fig. 3C). The spasticity quality of evidence was estimated as low performing using the GRADE system (based on risk of bias and imprecision) (Table 2).
Effects of RAGT on balance
Balance, measured using the BBS, was reported in three studies. The overall meta-analysis showed no significant difference (MD, 0.44; 95% CI, -0.18 to 1.06; I2=33.42%; p=0.16). Subgroup analysis by robot type showed no significant difference in the Lokomat, but a statistically significant improvement was observed for end-effector robot (MD, 0.95; 95% CI, 0.17–1.72; I2=uncheckable; p=0.02; Fig. 3D). The balance quality of evidence was estimated as low performing using the GRADE system (based on inconsistency and imprecision) (Table 2).
Effects of RAGT on walking ability
Ten studies reported the WISCI, and the overall meta-analysis showed significant improvement in the robot group (MD, 0.31; 95% CI, 0.07–0.55; I2=30.67%; p=0.01). Subgroup analysis according to robot type showed no significant difference in the Lokomat, but there was statistically significant improvement in the end-effector and wearable robots, with a larger effect size for wearable robots (MD, 1.30; 95% CI, 0.35–2.25; I2=uncheckable; p=0.01; Fig. 4A). The WISCI quality of evidence was estimated to be moderate, according to the GRADE system (based on risk of bias) (Table 2).
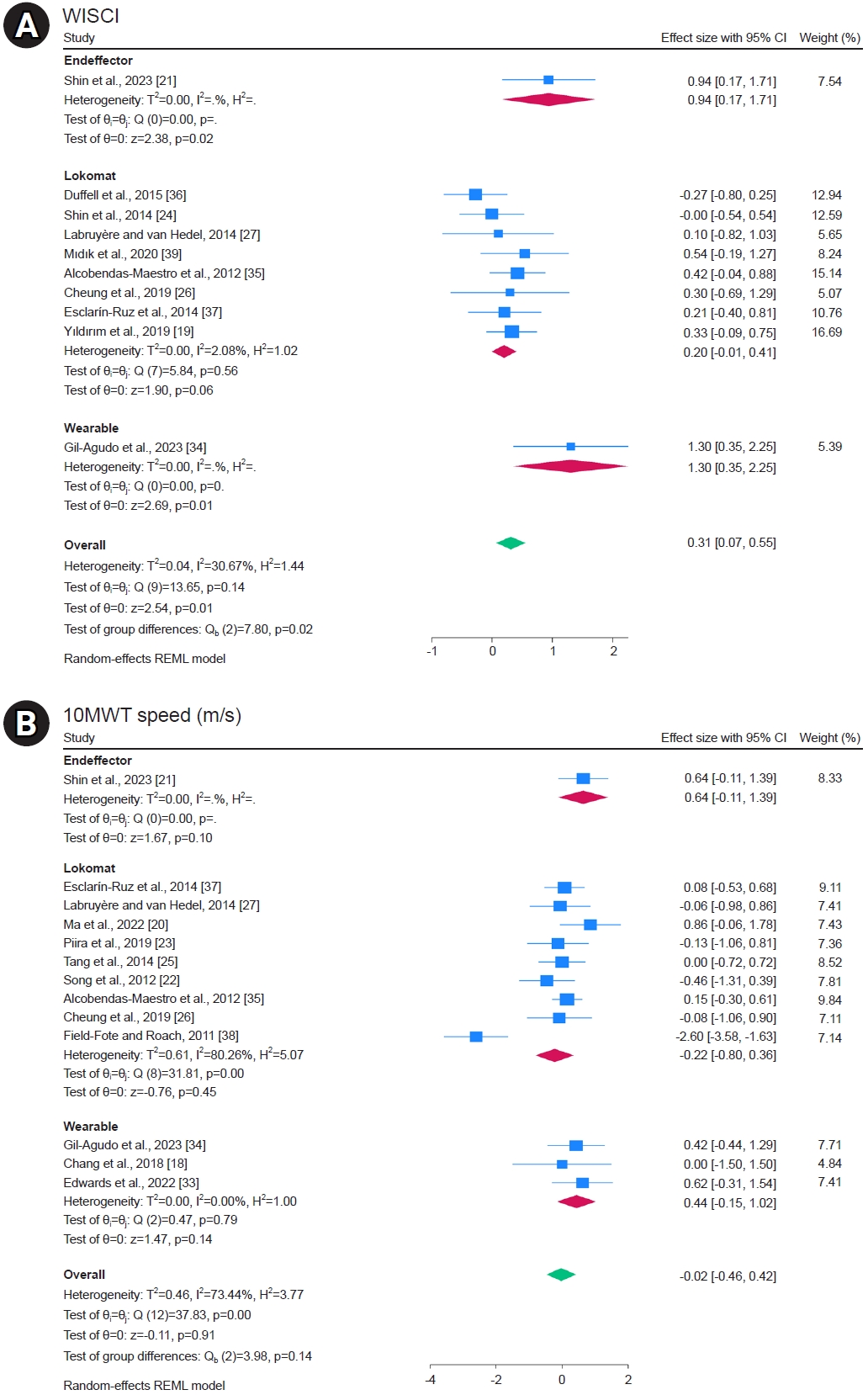
Meta-analysis of the effects of RAGT on walking ability in patients with spinal cord injury (SCI). (A) Walking index for spinal cord injury (WISCI). (B) 10-min walk test (10MWT) Speed (m/s).
Thirteen studies reported 10MWT Speed, and the overall meta-analysis showed no significant difference (MD, -0.02; 95% CI, -0.46 to 0.42; I2=73.44%; p=0.91). Subgroup analysis according to robot type showed no significant differences between the end-effector, Lokomat, and wearable robots (Fig. 4B). The 10MWT Speed quality of evidence was estimated to be very low when the GRADE system was used (based on risk of bias, inconsistency, indirectness, and imprecision) (Table 2).
Ten studies reported 6MWT distance, and the overall meta-analysis showed significant improvement in the robot group (MD, 0.38; 95% CI, 0.14–0.63; I2=0%; p=0.00). Subgroup analysis according to robot type showed no significant difference in the end-effector and wearable robots, but a statistically significant improvement was observed in the Lokomat (MD, 0.40; 95% CI, 0.10–0.70; I2=0%; p=0.01; Fig. 5A). The 6MWT distance quality of evidence was estimated to be moderate, according to the GRADE system (based on risk of bias) (Table 2).

Meta-analysis of the effects of the RAGT on walking ability in patients with spinal cord injury (SCI). (A) 6-min walk test (6MWT) Distance (m). (B) Step length (m).
Two studies reported step length, and the overall meta-analysis showed no significant difference (MD, -0.15; 95% CI, -0.84 to 0.55; I2=0%; p=0.68). Subgroup analysis according to robot type showed no significant differences between the Lokomat and wearable robots (Fig. 5B). The step length quality of evidence was estimated to be low when using the GRADE system (based on imprecision and publication bias) (Table 2).
Effects of RAGT on body composition
Two studies reported body mass, and the overall meta-analysis showed no significant difference (MD, 0.02; 95% CI, -0.67 to 0.71; I2=0%; p=0.96). Two studies reported lean mass, and the overall meta-analysis showed no significant difference (MD, 0.02; 95% CI, -0.67 to 0.72; I2=0%; p=0.95). Two studies reported fat tissue percentage, and the overall meta-analysis showed no significant difference (MD, 0.09; 95% CI, -0.60 to 0.79; I2=0%; p=0.79). The quality of evidence for each sub-analysis of body composition was rated as moderate (based on imprecision) using the GRADE system (Fig. 6A, Table 2).
Effects of RAGT on cardiopulmonary function
Two studies reported FEV1, and the overall meta-analysis showed no significant difference (MD, 0.49; 95% CI, -0.20 to 1.18; I2=0%; p=0.17). Two studies reported FVC, and the overall meta-analysis showed no significant difference (MD, 0.33; 95% CI, -0.35 to 1.02; I2=0%; p=0.34). Two studies reported heart rate, and the overall meta-analysis showed no significant difference (MD, 0.56; 95% CI, -0.17 to 1.30; I2=10.09%; p=0.13). Two studies reported PCF, and the overall meta-analysis showed no significant difference (MD, 0.26; 95% CI, -0.41 to 0.94; I2=0%; p=0.44). In the sub-analysis of cardiopulmonary function, the quality of evidence was assessed using the GRADE system, with only peak expiratory flow rated low (based on imprecision and publication bias) and the rest rated moderate (based on imprecision) (Fig. 6B, Table 2).
Subgroup analysis
Subgroup analyses were performed based on intervention period and onset duration (Supplementary Fig. S2, Table 3). Subgroup analysis according to intervention duration showed significant improvements in activities of daily living difference (SMD, 0.34; 95% CI, 0.09–0.60; I2=0%; p=0.01; Supplementary Fig. S2A) and WISCI (MD, 0.33; 95% CI, 0.07–0.60; I2=0%; p=0.01; Supplementary Fig. S2E) in the group that intervened for >2 months. The 6MWT distance showed significant improvement in intervention periods of <1 month (MD, 0.59; 95% CI, 0.10–1.07; I2=23.92%; p=0.02) and >2 months (MD, 0.38; 95% CI, 0.04–0.72; I2=0%; p=0.03; Supplementary Fig. S2I).
Onset duration was categorized as acute (0–3 months), subacute (4–12 months), and chronic (>12 months). When sub-analyzed according to onset duration, patients in the subacute phase had significant improvements in activities of daily living (SMD, 0.37; 95% CI, 0.03–0.70; I2=0%; p=0.03; Supplementary Fig. S2B) and WISCI (MD, 0.51; 95% CI, 0.06–0.96; I2=35.72%; p=0.03; Supplementary Fig. S2F). The 6MWT distance showed significant improvement in acute patients (MD, 0.69; 95% CI, 0.06–1.32; I2=38.51%; p=0.03; Supplementary Fig. S2J).
Adverse events
Six [18,26-30] of the 23 studies reported no adverse events. A variety of adverse events were reported in five studies [23,31-34], of which four [23,31,33,34] reported skin abrasions at the site of the robot, which improved with the application of pads at the time of treatment. Additionally, three studies [32-34] reported musculoskeletal pain, particularly knee and neck pain; one study [33] reported possibly related numbness; and one study [34] reported fatigue. The remaining 12 studies did not mention safety or side effects.
DISCUSSION
This systematic review investigated the efficacy of RAGT in patients with SCI. A study showed a high level of evidence that RAGT significantly improves activities of daily living and muscle strength. RAGT has been shown to significantly improve walking ability, specifically WISCI and 6MWT distances. Spasticity, balance, body composition, and cardiopulmonary function did not differ significantly between RAGT and conventional rehabilitation groups. Subgroup analyses showed significant improvements in activities of daily living and WISCI in subacute patients with onset duration of 4–12 months and intervention duration of ≥2 months.
The mechanism by which RAGT improves functionality in patients with SCI involves reinforcing proprioceptive input to the spinal cord through walking-related rhythmic movements facilitated by robots. This process activates the central pattern generator embedded within the lumbosacral spinal cord and can induce plastic changes at the spinal cord level and within the sensorimotor cortex (S1 and S2) and cerebellar regions of the brain [41-44].
When analyzing the effects of robot type, the results were heterogeneous. For activities of daily living and muscular strength, the Lokomat had the largest effect size; for the WISCI, the wearable robot had the largest effect size; and for balance, the end effector had the largest effect size. Among existing studies, none compared the effects of the three types of robots simultaneously; however, studies comparing the Lokomat and wearable robots have shown that wearable robots show greater improvement in walking speed [10]. In previous studies, the difference has been explained between wearable robots and stationary treadmill systems, such as the Lokomat, because the former is an overground walking system that requires patients to exert greater effort on their trunk and arms while engaging in higher cognitive and cardiovascular efforts [45]. This increased effort results in elevated muscle activity in the torso and pelvic floor [46], significantly enhancing motor and sensory functions [47]. Additionally, wearable robots provide more proprioceptive stimulation and adaptability to real-world environments through overground walking programs, thereby promoting greater neuroplasticity and facilitating connectivity remodulation [48]. The findings suggest that caution is needed in generalizing the efficacy of end-effector robots in improving balance, owing to limited studies. However, unlike exoskeleton-type robots, end-effector robots allow the knee and hip joints of participants to move freely, while their feet remain attached to the robot’s footplates. Previous studies have shown that this unrestricted movement of the hip and knee joints encourages destabilization training and promotes the coordination of postural control through feedback and feed-forward mechanisms, ultimately improving overall postural control [49].
Typically, approximately half of the recovery occurs within the initial 2 months following an injury, with a declining recovery rate over the subsequent 4 months. According to the 1-year mark post-injury, neurological recovery is generally close to completion [50]. Engaging in repetitive functional gait training during the acute phase aids in restoring muscle activation and relearning proper gait patterns. However, individuals with chronic SCI face challenges due to a slower rate of neuroplasticity, making motor relearning and reducing dependence on walking aids more challenging. The sub-analysis results of this study can also be said to support the above hypothesis because there was a significant improvement in the activities of daily living and WISCI in subacute patients with an onset period of 4–12 months. However, in this analysis, RAGT was more effective in the subacute phase than in the acute phase because, firstly, in the acute phase, there is a lack of medical stability and a higher complication rate [51], which may reduce the effectiveness of RAGT, which is a high-intensity repetitive exercise, and, secondly, because of the difference in treatment duration between the two groups. Previous subgroup analyses have shown that RAGT is more effective when the treatment duration is 2 months or more, but 71.4% of the trials in the acute phase had a treatment duration of less than 1 month, and only 25% of the trials in the subacute phase had a treatment duration of less than 1 month, which may explain the smaller effect analyzed in the acute phase. Future studies comparing treatment effects in groups of patients with the same treatment duration and protocol but different onset times are needed.
Strengths and limitations
The strength of this meta-analysis is that it provided the most up-to-date and comprehensive information on the efficacy of RAGT in individuals with SCI. Additionally, we sub-analyzed the effects of robot type, duration of intervention, and onset of SCI.
This meta-analysis has several limitations. First, there was a limited pool of involved articles, with the majority having small sample sizes. The included papers varied according to robot type: 16 Lokomat papers, 6 wearable robot papers, and 1 end-effector robot paper. The largest sample size of the included papers was 88, and the remaining papers all had small sample sizes of less than 100. Previous research has shown that pilot studies or studies with small sample sizes can inflate pooled effect sizes, even in meta-analyses [52], so this study should be interpreted with this in mind. Second, heterogeneity was observed among the included studies. For the primary outcome, the 10MWT speed meta-analysis was highly heterogeneous, with an I2 value of 73.44%. This was because the study protocols varied according to robot type, intervention duration, treatment time, robot body weight support percentage, guidance force, and speed, and the injury level, severity, and onset time of the included patients varied. To address this heterogeneity, we performed subgroup analyses based on the duration of intervention and onset. Third, it does not take into account initial ASIA assessment results, level of injury, and patient age. Studies have shown that recovery and ambulation in SCI patients are associated with initial ASIA assessment results and age, and the applicability of RAGT varies depending on whether the patient is a paraplegia or tetraplegia [53]. However, none of the RCTs published to date compared patients according to ASIA grades and level of injury, so a meta-analysis could not be performed. Further studies are needed to address this issue.
CONCLUSION
The meta-analysis findings indicated that RAGT resulted in beneficial enhancements in the activities of daily living, muscular strength, and walking ability. Notably, these improvements were more prominent in interventions lasting >2 months and among subacute patients. Consequently, RAGT appears to be a viable option for individuals with SCI, with positive outcomes without significant adverse events.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING INFORMATION
This study was supported by the Special Research Fund for ARM Development of the Korean Academy of Rehabilitation Medicine in 2023.
AUTHOR CONTRIBUTION
Conceptualization: Park JM, Shin JC. Methodology: Park JM. Formal analysis: Park JM. Funding acquisition: Shin JC. Project administration: Park JM. Visualization: Park JM. Writing – original draft: Park JM. Writing – review and editing: Park JM, Kim YW, Lee SJ, Shin JC. Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5535/arm.230039.
Supplementary Material S1.
PRISMA 2020 Checklist
Supplementary Material S2.
Search strategy
Supplementary Fig. S1.
Funnel plot to detect publication bias. WISCI, walking index for spinal cord injury; 10MWT, 10 meter walk test; 6MWT, 6 min walk test; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; PCF, peak cough flow; SMD, standardized mean difference.
Supplementary Fig. S2.
Subgroup analysis.
(A) Subgroup analysis of the effects of activities of daily living according to intervention period. (B) Subgroup analysis of the effects of activities of daily living according to onset period. (C) Subgroup analysis of the effects of muscular strength according to intervention period. (D) Subgroup analysis of the effects of muscular strength according to onset period. (E) Subgroup analysis of the effects of WISCI (walking index for spinal cord injury) according to intervention period. (F) Subgroup analysis of the effects of WISCI (walking index for spinal cord injury) according to onset period. (G) Subgroup analysis of the effects of 10MWT (10 meter walk test) speed according to intervention period. (H) Subgroup analysis of the effects of 10MWT (10 meter walk test) speed according to onset period. (I) Subgroup analysis of the effects of 6MWT (6 min walk test) distance according to intervention period. (J) Subgroup analysis of the effects of 6MWT (6 min walk test) distance according to onset period.