- Search
| Ann Rehabil Med > Volume 46(4); 2022 > Article |
|
Abstract
Cancer rehabilitation aims to enable patients to maximize their physical, social, psychological, and vocational functions within the limits that arise during the course of the disease and its treatment. According to recent domestic studies, most patients report one or more physical problems during or after cancer treatment. This review presents the latest updates on cancer-related rehabilitation issues. Cancer rehabilitation in Korea still faces various barriers, including a lack of awareness, problems with the healthcare delivery system, and high costs, and recognizing the need for rehabilitation during cancer treatment varies among patients and even physicians. Hence, an appropriate cooperative referral system for cancer rehabilitation requires improvement. We herein review the current status of and barriers to cancer rehabilitation in South Korea to resolve the issues of domestic cancer rehabilitation.
The Korean Cancer Society estimated that up to 260,000 Koreans might be newly diagnosed with cancer in 2021, with more than 2 million cancer survivors in Korea today. As the 5-year survival rates of all cancers have increased over the past 40 years, cancer patients have a longer life span, and more minor chronic illnesses after various cancer treatments have garnered growing attention [1,2]. The increase in the number of cancer survivors suggests to the necessity for recognizing their quality of life (QOL) and the need for rehabilitation throughout the treatment period [3].
Cancer rehabilitation allows patients to achieve optimal physical, social, psychological, and vocational functions within the limits imposed by cancer and its treatment [4]. According to a study that identified the patients’ recognition of rehabilitation services in South Korea, 87% of participants reported one or more physical problems during or after cancer treatment. The overall rehabilitation rate related to physical problems was 77% [5]. Recognizing the need for rehabilitation during cancer treatment varies among patients and even physicians. An appropriate cooperative referral system for cancer rehabilitation requires improvement [6].
The Cancer Rehabilitation Society was established in South Korea in 1994. With the accumulation of various clinical experiences, treatment-related disabilities and the rehabilitation of multiple types of cancer have been extensively researched. Moreover, cancer rehabilitation has recently been extended from pre-habilitation to advanced cancer-supportive care [7].
The cancer rehabilitation system and recognition among clinicians and patients differ markedly between countries [8]. Factors prohibiting rehabilitation treatment despite its demand include not only the absence of the appropriate referring system, but also the fact that clinicians do not recognize the need for rehabilitation and do not adequately prescribe or connect it [5]. According to a nationwide survey of cancer center programs in Korea, the types and methods of rehabilitation provided by cancer centers significantly differed based on regions and cancer type [9,10]. For example, more than 60% of patients with breast cancer, brain tumors, and spinal tumors receive rehabilitation services. Nevertheless, nearly 90% of gynecological, colorectal, or prostate cancer patients reported never or rarely having received rehabilitation. Moreover, many physiatrists have noticed the importance of mobilization and exercise during cancer treatment, but only a small portion have prescribed this rehabilitation program to their patients [10].
The Korean government’s 5-year National Cancer Control Plan (2021–2025) emphasizes the necessity of establishing a comprehensive support system for cancer survivors to lead a healthy life in the local community. Accordingly, a plan was prepared to establish a step-by-step approach to cancer survivorship care, including rehabilitation. Therefore, it is necessary to understand the current status of cancer patients and their rehabilitation needs and tackle the barriers associated with cancer rehabilitation in Korea. As the field of cancer rehabilitation is expanding and the government is focusing on managing patients with cancer, we herein identified the current status and difficulties of cancer rehabilitation in South Korea.
When Koreans survive to their average life expectancy of 83 years, their probability of getting cancer is 38.9%; two of five men aged 80 years (39.9%) and one in three women aged 87 years (35.8%) are estimated to have cancer [11]. Thyroid cancer was the most common cancer in Korea in 2019, followed by lung, stomach, colorectal, breast, prostate, and liver cancer. In men, lung cancer is the most prevalent, followed by stomach, colon, prostate, and liver cancers, whereas in women, breast cancer is the most common, followed by thyroid, colorectal, stomach, and lung cancers (Table 1). The incidence of breast, prostate, pancreatic, and kidney cancers has increased continuously since 1999. Among the top 10 cancers, thyroid, stomach, colon, breast, prostate, and kidney cancers have 10-year relative survival rates of over 70%. Therefore, it is essential to support cancer survivors to ensure that they can live a dignified life and improve their QOL within the limitations of body function and activity [12]. The difficulties experienced by patients vary depending on the primary cancer lesion and treatment details (including surgery, radiation therapy, and chemotherapy); therefore, it is crucial to understand the unmet needs of each patient. In this review, considering the prevalence of cancer in Korea, we focused on areas with a high demand for rehabilitation or a significant impact on rehabilitation efficacy.
In a Norwegian study, 63% of 1,325 respondents reported needing at least one rehabilitation service [13]. The three most requested requirements were the need for physical therapy (43%), physical training (34%), and psychological counseling (27%). However, other demands included support group sessions, admission to a convalescent home, consultation with a social worker, and occupational therapy. Similar results have been observed in the Korean population. More than 75% of cancer patients needing rehabilitation services for physical, psychological, and socioeconomic problems [5]. Furthermore, the needs for the services varied according to the type or stage of cancer, but only 18.2% of patients with physical problems and 5.3% with psychological and socioeconomic problems received such services. Among the three aforementioned areas, there were many complaints of physical problems, and nearly 90% of patients complained of one or more symptoms. The physical symptoms the patients complained of, from the most to least frequent, were as follows: fatigue, pain, numbness, weakness, limitation of range of motion, cognitive problems, lymphedema, respiratory problems, swallowing difficulty, and speech-language problems. Moreover, these problems could occur during or after cancer treatment. Rehabilitation therapies for physical problems include physical and occupational therapy, exercise education, pain control (including modalities, medication, and injection), lymphedema therapy, dysphagia therapy, speech and language therapy, cognitive therapy, and pulmonary rehabilitation. However, a helpful rehabilitation strategy for these symptoms was not provided satisfactorily. Only the degree of lymphedema or pain was recognized in only 36%–37% of cases, while other forms of management were far less recognized.
One barrier to satisfy the unmet needs of patients is the underdiagnosis and under-documentation of functional impairments, which leads to fewer referrals to specialists [14]. Likewise, cancer rehabilitation in Korea still faces many issues and barriers due to a lack of awareness and problems with the healthcare delivery system and the cost of medical services [10]. Although some patients are aware of rehabilitation treatment, the leading cause of their failure to receive rehabilitation therapy is the failure of medical staff to connect to them or the lack of hospitals equipped to provide rehabilitation treatment in their proximity [5] (Fig. 1). This finding suggests the necessity of resolving the issues of domestic cancer rehabilitation and medical policies.
The most common symptoms included fatigue, pain, numbness, joint limitation of range of motion, and weakness, with no significant differences between cancer types. However, cognitive, respiratory, swallowing, speech, and language problems and lymphedema differed in their frequency between cancer types [5]. The latest updates on some cancer-related rehabilitation issues are listed below.
Cancer-related fatigue is a distressing, persistent, and subjective sense of physical, emotional, and cognitive tiredness or exhaustion related to cancer or its treatment. It is not proportional to recent activity and interferes with normal functioning [15,16]. According to the National Comprehensive Cancer Network (NCCN) guidelines [16], all patients with cancer should be inquired about symptoms of fatigue at regular intervals during and after treatment. Fatigue management in cancer patients is complex because many factors can contribute to the symptoms. and managing these factors may reduce the symptoms [17]. Relatively limited evidence is available for pharmacological interventions, including psychostimulants, antidepressants, and supplements, whereas non-pharmacological interventions, including psychoeducation, cognitive behavior, and exercise programs, have shown positive and promising results [18–20]. Although many positive impacts on the effect of specific exercises based on cancer type have also been reported, a consensus protocol for exercise is needed. According to recommendations for patients with cancer, aerobic exercise at an intensity of 60%–80% of the maximum heart rate three times a week for 30 minutes or more, moderate intensity exercise for 150 minutes or more per week, or vigorous exercise of 75 minutes or more per week may reduce cancer-related fatigue. Moreover, resistance exercise twice a week for major muscle groups at moderate-to-vigorous intensity can be helpful [21,22]. Multimodal interventions, including exercise combined with other interventions such as high-intensity cardiovascular and heavy resistance training, relaxation, body awareness training, and massage, can also reduce fatigue [16].
Lymphedema is one of the most common complications in cancer patients and a common cause of referral to the rehabilitation department. It is the local or systemic swelling of the body caused by a decrease in the lymphatic transport capacity due to damage or dysfunction of the lymphatic system resulting from lymph node dissection during cancer treatment [23]. Surveilling the incidence of lymphedema in domestic patients with breast cancer for an average of 5 years or more showed that approximately 50% of patients developed lymphedema approximately 6 months postoperatively, and by 3 years, most patients showed symptoms [24]. Therefore, it is important to follow-up on the occurrence of lymphedema during this period as a study reported that postoperative surveillance can reduce the incidence rate [25].
The Korean Society of Lymphedema has published clinical guidelines for evidence-based universal standard practice for lymphedema after cancer treatment [26]. These guidelines recommend complex decongestive physical therapy for the effective treatment of lymphedema. Generally, the treatment comprises an intensive treatment period of 2–6 weeks followed by a management period of several months. Additional intensive treatment may be administered depending on the course of lymphedema. Compression bandages effectively reduce lymphedema, and the effect of treatment is amplified when accompanied by manual lymphatic drainage massage. Professionals recommend compression bandage treatment. The therapeutic effect of compression bandages alone does not significantly differ from that of self-exercise, self-massage, and intermittent pneumatic compression therapy. Therefore, compression bandaging alone can also be considered when considering the economic aspects. A manual lymphatic drainage massage performed by a professional therapist is effective for the treatment of lymphedema, but its efficacy improves with other therapies. Progressive low-intensity exercise does not worsen lymphedema or increase its incidence, yet it improves physical function and QOL. However, the effect of exercise on lymphedema reduction remains controversial.
Radiation fibrosis syndrome (RFS) is another frequently observed complication of radiation therapy [12]. Radiation can damage the spinal cord, plexus, nerve roots, peripheral nerves, and muscles within the radiation field. This is known as myelo-radiculo-plexo-neuromyopathy with multiple clinical symptoms, including progressive myelopathy and plexopathy with gait disturbance due to weakness, shoulder dysfunction, and limited range of motion. The typical neuromuscular symptom is dropped head syndrome, which induces neck extension, weakness, pain, and dysphagia. The most critical role of rehabilitation physicians in the care of RFS is the identification, evaluation, and proper treatment prescription. Currently, this phenomenon cannot be slowed down or reversed. Although the current evidence is limited, supportive care, including physical therapy, medications, and bracing, could alleviate disturbances in patients with RFS [27].
Damage to the peripheral nerve fibers after neurotoxic chemotherapy usually affects sensory nerve fibers, but motor or autonomic neuropathic symptoms may occur depending on the type of anticancer drug. Most agents are neurotoxic in a dose-dependent manner, with either a single high dose or an accumulative dose. If chemotherapy- induced peripheral neuropathy (CIPN) is suspected, the use of taxanes, platinum drugs, vinca alkaloids, thalidomide, and bortezomib should be considered. The drug-specific clinical presentations and neurotoxicity of CIPN are summarized in Table 2 [28]. Moreover, it is crucial to exclude other causes that may lead to neuropathy, including tumor recurrence, para-neoplastic syndrome, atherosclerotic ischemic disease, thyroid dysfunction, and vitamin B12 deficiency [28]. Although these sensory and motor symptoms may be alleviated over time, some may persist long-term, leading to inappropriate proprioceptive feedback, postural control, higher fall risk, and a compromised QOL. As CIPN can continuously produce a significant burden of suffering for cancer survivors, the appropriate management of CIPN is necessary, even if it is very complex and challenging. Unfortunately, consensus on the treatment and prevention of CIPN remains insufficient in the recent guidelines of the American Society of Clinical Oncology [29]. The guidelines suggest that the use of duloxetine may be worthwhile for treatment, but present a relatively conservative recommendation regarding tricyclic antidepressants, gabapentin, and topical gel treatments due to insufficient medical evidence. Notably, various sensorimotor-based exercises can ameliorate CIPN symptoms, thereby improving postural stability. Long-term exercise (≥8 weeks) is also reported to be more effective than short-term exercise. However, additional evidence regarding the exercise protocol is necessary [29].
According to the NCCN, at least 5 years of endocrine therapy is recommended for breast cancer patients if the cancer is hormone receptor-positive from stage 0, the noninvasive stage. Tamoxifen is recommended as the first-line medication for premenopausal patients, whereas an aromatase inhibitor (AI) is recommended instead of tamoxifen in postmenopausal patients over 60 years of age. Otherwise, these drugs are associated with a significantly higher incidence of musculoskeletal problems during treatment. Up to 80% of patients complain of AI-related musculoskeletal symptoms, which comprise a large proportion of patients taking tamoxifen (i.e., approximately 30%). Many even decide to stop taking AI owing to such symptoms, which significantly affects their QOL. The discontinuation of endocrine therapy also increases the risk of cancer recurrence. Therefore, relieving the pain of patients and enabling them to continue treatment are extremely important as they will not only improve the patients’ QOL, but also extend their lifespan.
These symptoms usually emerge 6–8 weeks after AI treatment and may worsen for a year and subsequently stabilize. Symptoms may appear as various forms of pain in the joints, tendons, and muscles. Notably, AI-related musculoskeletal symptoms do not induce neuropathy. Nonsteroidal anti-inflammatory drugs (NSAID) and acetaminophen are reportedly effective in 60%–70% of patients, and additional prescriptions may be considered. A Cochrane review in 2020 stated that the evidence of the effect of exercise therapy on pain, stiffness score, grip strength, health-related QOL score, and adherence to AI is uncertain [30]. However, as several randomized controlled trial studies have reported some positive effects of exercise programs, including aerobic exercise and strength training [31–33], it is also necessary to continuously apply it to patients and establish additional evidence. If the treatments do not harm the patient, it is necessary to accumulate evidence by actively adding potentially beneficial treatments to the comprehensive care strategy.
This review focuses on cancer statistics in South Korea and recently updated cancer-related rehabilitation issues. Most medical staff recognize the need for treatment in rehabilitation medicine. Otherwise, cancer rehabilitation still encounters many barriers owing to a lack of awareness, problems with the healthcare delivery system, and medical costs. In this review, we provide an overview of this problem to improve the status of cancer rehabilitation to appropriately manage the functional limitations imposed by cancer and its treatment in South Korea.
AUTHOR CONTRIBUTION
Conceptualization: Hong BY, Yoon J. Methodology: Hong BY, Yoon J. Project administration: Hong BY, Yoon J. Visualization: Hong BY, Yoon J. Writing — original draft: Hong BY, Yoon J. Writing — review and editing: Hong BY, Yoon J. Approval of final manuscript: all authors.
Fig. 1
Unmet needs (experience of rehabilitation/demand for rehabilitation) of patients with actual rehabilitation problems. Adapted from Jo et al. J Korean Acad Rehabil Med 2010;34:691–700 [5].
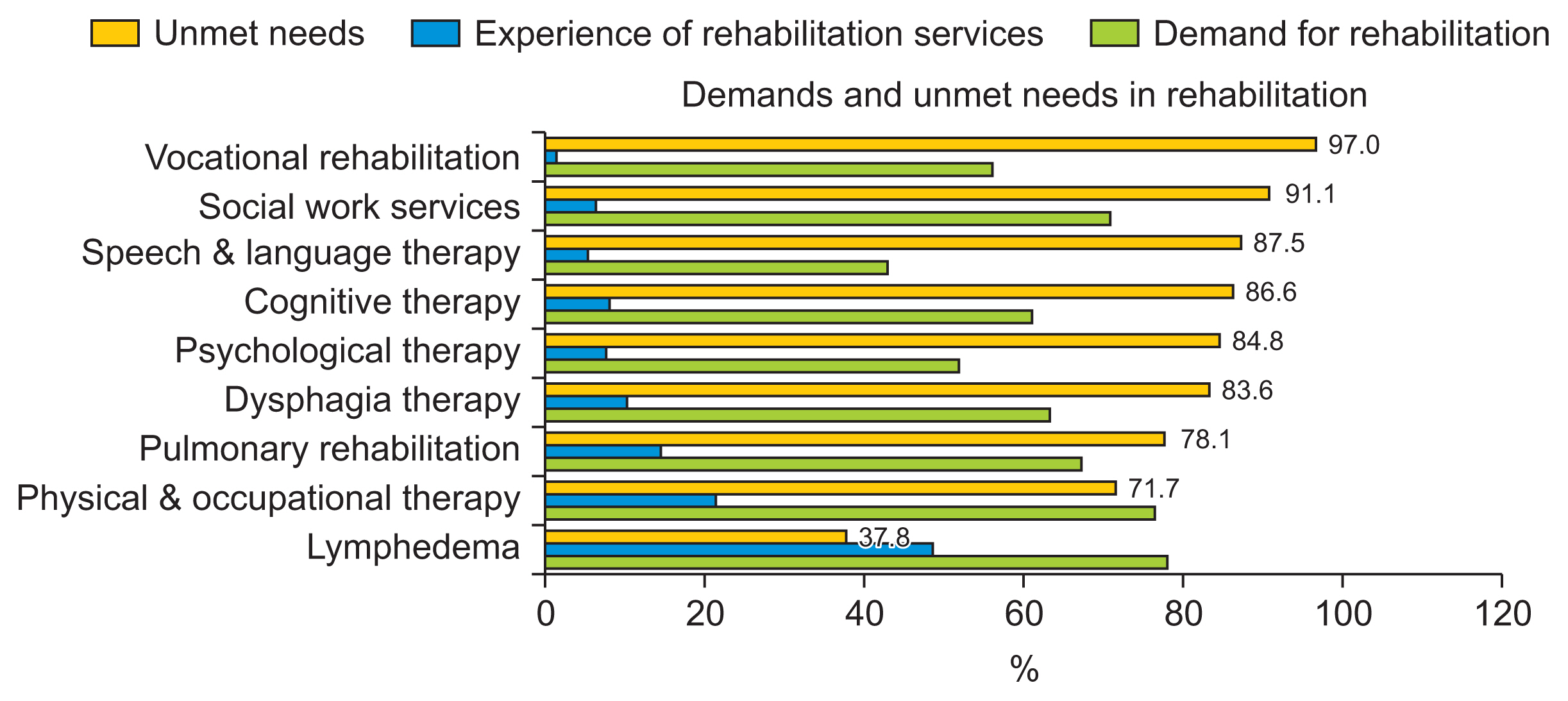
Table 1
Crude and age-standardized cancer incidence rates by sex in Korea, 2019
Adapted from the National Cancer Information Center (https://www.cancer.go.kr).
Table 2
Chemotherapeutic agents that cause peripheral neuropathies and associated features
REFERENCES
1. Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat 2021;53:316-22.




2. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat 2022;54:330-44.




3. Movsas SB, Chang VT, Tunkel RS, Shah VV, Ryan LS, Millis SR. Rehabilitation needs of an inpatient medical oncology unit. Arch Phys Med Rehabil 2003;84:1642-6.

4. Cromes GF Jr. Implementation of interdisciplinary cancer rehabilitation. Rehabil Couns Bull 1978;21:230-7.
5. Jo JM, Hwang JH, Lee CH, Kang HJ, Yu JN. The need of cancer patients for rehabilitation services. J Korean Acad Rehabil Med 2010;34:691-700.
7. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 2013;92:715-27.

8. New PW, Townson A, Scivoletto G, Post MW, Eriks-Hoogland I, Gupta A, et al. International comparison of the organisation of rehabilitation services and systems of care for patients with spinal cord injury. Spinal Cord 2013;51:33-9.



9. Kim JY, Yi ES. Nationwide survey of cancer center programs in Korea. J Exerc Rehabil 2017;13:300-6.




10. Yang EJ, Chung SH, Jeon JY, Seo KS, Shin HI, Hwang JH, et al. Current practice and barriers in cancer rehabilitation: perspectives of Korean physiatrists. Cancer Res Treat 2015;47:370-8.




11. National Cancer Information Center. Cancer statistics [Internet] Goyang, Korea, National Cancer Information Center. 2020;[cited 2022 Aug 5]. Available from: https://www.cancer.go.kr/
.
13. Thorsen L, Gjerset GM, Loge JH, Kiserud CE, Skovlund E, Flotten T, et al. Cancer patients’ needs for rehabilitation services. Acta Oncol 2011;50:212-22.


14. Cheville AL, Beck LA, Petersen TL, Marks RS, Gamble GL. The detection and treatment of cancer-related functional problems in an outpatient setting. Support Care Cancer 2009;17:61-7.



15. Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597-609.




16. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw 2015;13:1012-39.



17. Mortimer JE, Barsevick AM, Bennett CL, Berger AM, Cleeland C, DeVader SR, et al. Studying cancer-related fatigue: report of the NCCN Scientific Research Committee. J Natl Compr Canc Netw 2010;8:1331-9.


18. Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 2017;3:961-8.



19. Mitchell SA, Hoffman AJ, Clark JC, DeGennaro RM, Poirier P, Robinson CB, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs 2014;18:Suppl. 38-58.


21. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409-26.


22. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin 2016;66:43-73.



24. Kim M, Park IH, Lee KS, Ro J, Jung SY, Lee S, et al. Breast cancer-related lymphedema after neoadjuvant chemotherapy. Cancer Res Treat 2015;47:416-23.




25. Yang EJ, Ahn S, Kim EK, Kang E, Park Y, Lim JY, et al. Use of a prospective surveillance model to prevent breast cancer treatment-related lymphedema: a single-center experience. Breast Cancer Res Treat 2016;160:269-76.




26. Korean Society of Lymphedema. Clinical guideline for secondary lymphedema. Seoul, Korea: Korean Society of Lymphedema; 2015.
27. Stubblefield MD. Clinical evaluation and management of radiation fibrosis syndrome. Phys Med Rehabil Clin N Am 2017;28:89-100.


28. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 2017;81:772-81.




29. Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:1941-67.


30. Roberts KE, Rickett K, Feng S, Vagenas D, Woodward NE. Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev 2020;1:CD012988.



31. Paulo TR, Rossi FE, Viezel J, Tosello GT, Seidinger SC, Simoes RR, et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes 2019;17:17.




32. Gentry AL, Erickson KI, Sereika SM, Casillo FE, Crisafio ME, Donahue PT, et al. Protocol for Exercise Program in Cancer and Cognition (EPICC): a randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy. Contemp Clin Trials 2018;67:109-15.



- TOOLS
-
METRICS

- Related articles in ARM
-
Fact Sheet on Cardiac Rehabilitation for Cardiovascular Disease in South Korea2023 October;47(5)
Spinal Cord Injury Fact Sheet in Korea2023 February;47(1)
Correction: Stroke Rehabilitation Fact Sheet in Korea2022 April;46(2)
Stroke Rehabilitation Fact Sheet in Korea2022 February;46(1)
Clinical Practice Guideline for Cardiac Rehabilitation in Korea2019 June;43(3)






