- Search
| Ann Rehabil Med > Volume 44(4); 2020 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
AUTHOR CONTRIBUTION
Conceptualization: Han MH, Kim MW. Methodology: Han MH, Leigh JH. Formal analysis: Han MH, Kim DY. Visualization: Han MH. Writing - original draft: Han MH. Writing - review and editing: Han MH, Leigh JH, Kim MW. Approval of final manuscript: all authors.
Fig. 1.
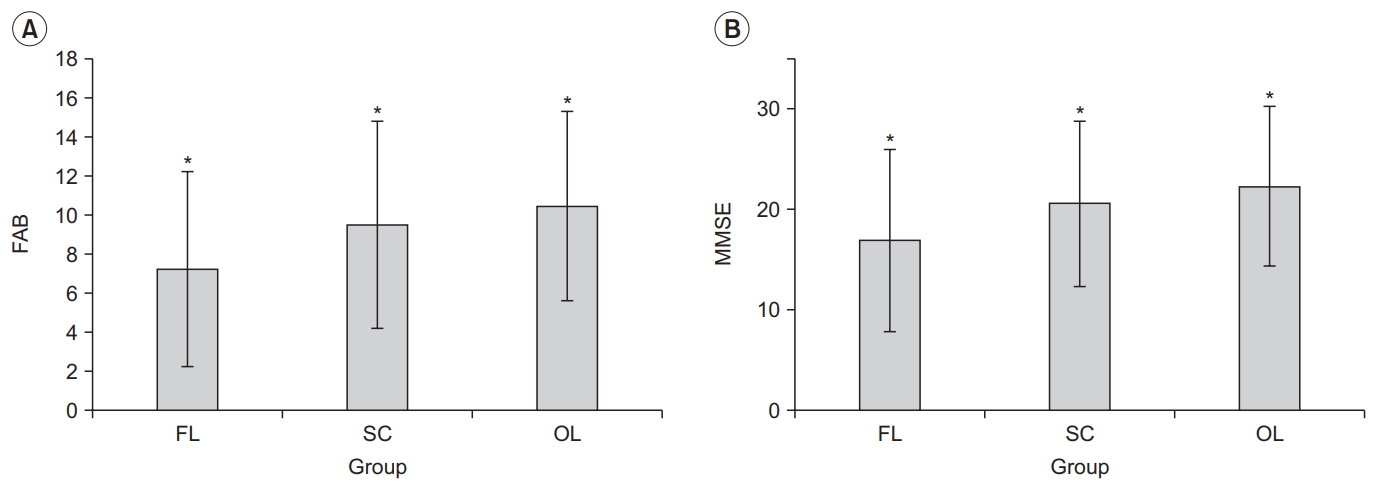
Fig. 2.
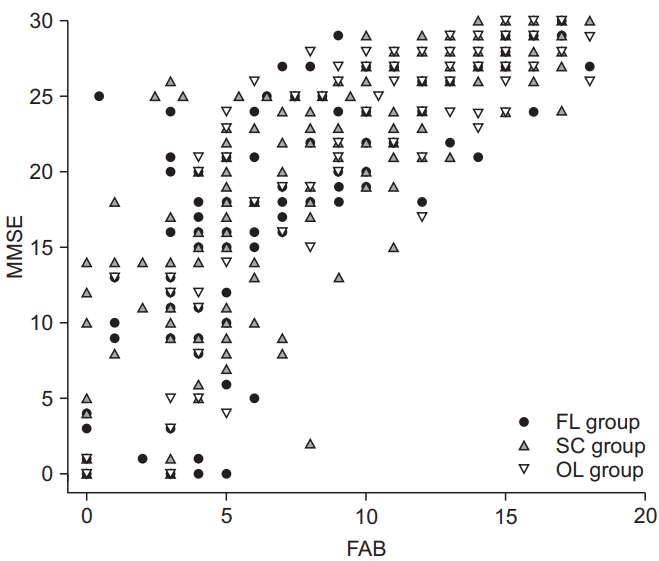
Table 1.
Table 2.
| Lesions involving the frontal lobe cortex | Lesions related to the frontal subcortical circuit | Other lesions | |
|---|---|---|---|
| FAB score | |||
| Totala) | 7.28±4.99b,e) | 9.53±5.31 | 10.48±4.83 |
| Similaritiesa) | 0.95±1.08f) | 1.27±1.03 | 1.37±1.00 |
| Lexical fluencya) | 0.45±0.77f) | 0.72±0.85 | 0.79±0.87 |
| Motor seriesa) | 1.23±1.29b,e) | 1.73±1.26 | 1.88±1.22 |
| Conflicting instructionsa) | 1.19±1.17c,e) | 1.71±1.25 | 1.90±1.28 |
| Go-no-goa) | 1.05±1.09b,e) | 1.48±1.19 | 1.64±1.15 |
| Prehension behavioura) | 2.36±1.14b,f) | 2.6 3± 0.93 | 2.89±0.52 |
| MMSE | |||
| Totala) | 16.94±9.04b,e) | 20.68±8.38 | 22.29±7.92 |
| Three-stage commanda) | 1.96±1.06d,f) | 2.32±0.95 | 2.32±0.97 |
| Attention and calculationa) | 1.66±1.87b,e) | 2.36±1.93 | 2.89±1.98 |
| Language (repetition) | 0.70±0.46 | 0.81±0.41 | 0.81±0.40 |
Values are presented as mean±standard deviation.
FAB, Frontal Assessment Battery; MMSE, Mini-Mental State Examination; NIHSS, National Institutes of Health Stroke Scale; FL group, with lesions involving the frontal lobe cortex; SC group, with lesions related to the frontal subcortical circuit without frontal cortex involvement; OL group, with other lesions that were unrelated to the frontal subcortical circuit.
b) p<0.05 indicate a significantly different compared to SC and OL groups after controlling initial NIHSS score.
c) p<0.05 indicate a significantly different compared to OL group after controlling initial NIHSS score.
Table 3.
| Test | Number of patients | Total FAB score | Total MMSE score | Total FAB score with a partial correlation after controlling for the MMSE score |
|---|---|---|---|---|
| Visuo-motor coordination | ||||
| Trail making test A | 96 | -0.467a) | -0.314a) | -0.405a) |
| Trail making test B | 56 | -0.382b) | -0.223 | -0.394a) |
| High cognition function (executive function) test | ||||
| Card sorting test | 166 | 0.645a) | 0.606a) | 0.273a) |
| Memory test | ||||
| Digital span (forward) | 166 | 0.742a) | 0.711a) | 0.697a) |
| Digital span (backward) | 166 | 0.790a) | 0.765a) | 0.362a) |
| Verbal learning test (recall) | 163 | 0.702a) | 0.687a) | 0.690a) |
| Verbal learning test (interference) | 163 | 0.691a) | 0.703a) | 0.684a) |
| Verbal learning test (recognition) | 163 | 0.634a) | 0.711a) | 0.613a) |
| Attention test | ||||
| Visual CPT (correct response) | 164 | 0.563a) | 0.499a) | 0.576a) |
| Visual CPT (commission response) | 164 | -0.586a) | -0.521a) | -0.576a) |
| Visual CPT (reaction time) | 164 | -0.199b) | -0.209a) | -0.216a) |
| Auditory CPT (correct response) | 164 | 0.645a) | 0.606a) | 0.612a) |
| Auditory CPT (commission response) | 164 | -0.646a) | -0.609a) | -0.613a) |
| Auditory CPT (reaction time) | 164 | -0.046 | -0.017 | -0.283a) |
| Word-Color test A | 126 | -0.730a) | -0.598a) | -0.410a) |
| Word-Color test B | 126 | -0.637a) | -0.586a) | -0.315a) |
| Word-Color test C | 126 | -0.728a) | -0.664a) | -0.001 |
| Word-Color test D | 126 | -0.687 | -0.602a) | -0.356a) |
Table 4.
| Test | MMSE<24 (n=86) | MMSE≥24 (n=83) |
|---|---|---|
| Visuo-motor coordination | ||
| Trail making test A | -0.336 | -0.523a) |
| Trail making test B | -0.453 | -0.329a) |
| High cognition function (executive function) test | ||
| Card sorting test | 0.502a) | 0.402a) |
| Memory test | ||
| Digital span (forward) | 0.548a) | 0.610a) |
| Digital span (backward) | 0.683a) | 0.570a) |
| Verbal learning test (recall) | 0.626a) | 0.370a) |
| Verbal learning test (interference) | 0.574a) | 0.367a) |
| Verbal learning test (recognition) | 0.474a) | 0.305a) |
| Attention test | ||
| Visual CPT (correct response) | 0.396b) | 0.515a) |
| Visual CPT (commission response) | -0.396b) | -0.515a) |
| Visual CPT (reaction time) | -0.160 | -0.068 |
| Auditory CPT (correct response) | 0.430a) | 0.365a) |
| Auditory CPT (commission response) | -0.430a) | -0.368a) |
| Auditory CPT (reaction time) | -0.036 | -0.148 |
| Word-Color test A | -0.597a) | -0.611a) |
| Word-Color test B | -0.704a) | -0.458a) |
| Word-Color test C | -0.609a) | -0.569a) |
| Word-Color test D | -0.228 | -0.482a) |







