INTRODUCTION
Collet-Sicard syndrome is a condition showing unilateral paralysis of the lower cranial nerves (9, 10, 11 and 12). The most common causes of it are tumors in the skull base area and nasal part of the pharynx. Other causes include inflammatory disease, vascular disease and fracture.
1-
3
Although Jefferson fracture is a bursting fracture occurring in the 1
st cervical vertebra, it rarely induces cranial nerve damage. In Korea, 2 cases of Collet-Sicard syndromes due to tumor and thrombosis have been reported so far. No case of Collet-Sicard syndrome due to Jefferson fracture has been reported yet to the best of our knowledge.
4,
5 We describe here a case of Collet-Sicard syndrome, which developed after Jefferson fracture together with discussion of the relevant literature.
CASE REPORT
A 46-year old male patient was admitted to our hospital via the emergency room due to neck pain that occurred after falling from a height of 2 m. At the time of admission, level of consciousness and vital signs were normal without particular underlying diseases in the past.
Fracture in the 1
st cervical vertebra was found without malformations such as basilar invagination in the relevant tests including blood test, urine test, radiography, computed tomography (CT), magnetic resonance imaging (MRI) of cervical spine, and brain CT, all of which were performed for the purpose of differential diagnosis and to detect comorbidity (
Fig. 1-A). Physical and neurological examinations revealed decreased gag reflex in the left and deviation of the tongue to the left on protrusion.
At day 2 of admission, posterior fixation was performed from the occiput through C3 (
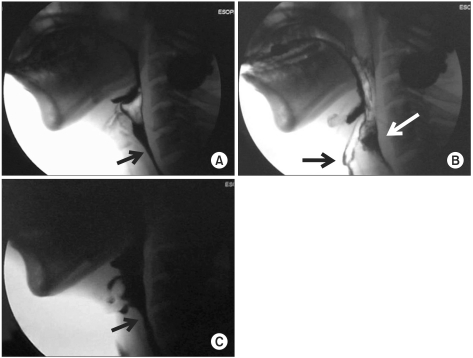
Fig. 1-B). At day 15 of admission, the patient complained of dysphagia and aspiration symptoms such as cough and choking during food swallowing. Thus, a videofluoroscopic swallowing study (VFSS) was performed. In the swallowing study, only the thick barium (thick liquid) study was possible, and further study could not be performed due to the risk of aspiration. It showed retention of food in the oral cavity after swallowing and delayed oral transit time. In addition, it was found that pharyngeal reflex was weak in the pharyngeal phase and movement of the pharyngeal wall was decreased. Test food rarely went below the pharyngoesophageal junction due to the poor relaxation of the upper esophageal sphincter (
Fig. 2-A) and most of them remained in the vallecula and pyriformis sinus, showing aspiration into the airway (
Fig. 2-B).
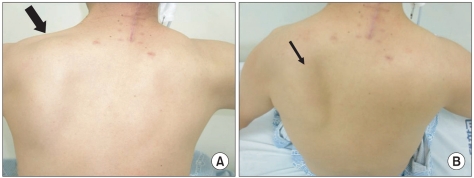
After the study, oral feeding was stopped and nasogastric tube feeding was initiated. The patient was transferred to the department of rehabilitation at day 17 of admission for rehabilitation treatment of dysphasia. Physical examination performed after the transfer showed decreased muscular strength of fair grade (grade III) in the flexors, extensors, abductors, and adductors of both shoulders. Other than that no particular finding were recorded, and the sensory function was normal. Atrophy of the left trapezius muscle and winged scapula in the left were observed (
Fig. 3).
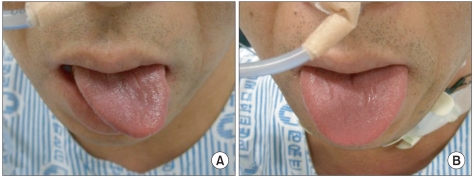
Neurological examination still showed limited laryngeal elevation, decreased gag reflex and deviation of tongue to the left on protrusion (
Fig. 4-A). A follow-up videofluoroscopic swallowing study performed at day 29 of admission showed similar results to that of the previous examinations.
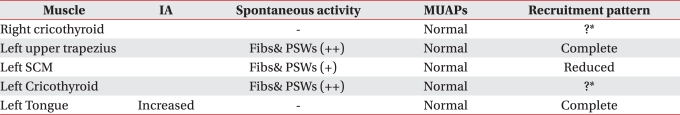
An electrodiagnostic study was performed at day 31 of admission to assess the degree of damage of the lower cranial nerves. Needle electromyography showed abnormal spontaneous activities such as positive sharp waves and fibrillation potentials in the cricothyroid muscle, trapezius muscle and sternocleidomastoideus. In addition, although polyphasic motor unit was not observed during muscular contraction, decreased recruitment patterns were observed, which led to the diagnosis of cranial neuropathy in the 9
th, 10
th and 12
th in the left (
Table 1). No paralysis of glottis was observed in the laryngeal endoscopic examination which was performed at day 32 of admission to assess the degree of paralysis of glottis due to damage of cranial nerves.
Swallowing rehabilitation treatments such as oral and pharyngolaryngeal muscle reinforcement exercise, swallowing refl ex exercise, functional electric stimulation therapy and ice cube swallowing were performed during the hospitalization in the department of rehabilitation. At day 40 of admission, laryngeal elevation improved and at day 50 deviation of tongue to the left on protrusion was improved (
Fig. 4-B). Follow-up VFSS performed at day 57 of admission showed normal pharyngeal reflex, improved, but a decreased movement of pharyngeal wall and the poor relaxation of upper esophageal sphincter. Also, there was massive aspiration finding, so the nasogastric tube feeding was preserved.
DISCUSSION
Fracture of C1 commonly occurs when vertical load is applied in the direction of cephalocaudal axis due to hitting the head on the ground in car accidents or diving,
6 and the incidence rate of the fracture of C1 is about 2-13% of the fractures of cervical vertebrae. About 33% of the fracture of C1 is Jefferson fracture. Clinical symptoms of fracture of C1 is nonspecific and mostly occur as laryngeal pain, headache, or neck stiffness.
7 Generally, however, the fracture of C1 does not accompany the damage of cranial nerve because spinal canal is wider than the spinal cord and bone fragment is pushed out, widening the spinal canal.
6 In some patients, dangerous complications occurring after the Jefferson fracture could occur as a result of the damage of spinal cord or vertebral artery, not cranial nerve.
7
Collet-Sicard syndrome is a condition which unilaterally paralyzes lower cranial nerves from 9
th through 12
th. The 9
th, 10
th and 11
th cranial nerves run through the jugular foramen and 12
th cranial nerve runs through the hypoglossal foramen of base of skull. Then, the 4 nerves run in close proximity between the styloid process of skull and 1
st cervical vertebra.
8
Zielinski et al., performed an autopsy and reported that the space between the transverse foramen and styloid process of C1 is just 8-10 mm, and lower cranial nerves (9
th, 10
th, 11
th and 12
th) that run through this space could be oppressed by the lateral displacement of C1 that accompany the basilar impression due to Jefferson fracture.
9
Hsu et al., observed that Collet-Sicard syndrome was accompanied in a patient with fracture of C1 in combination with congenital basilar impression, and that lower cranial nerves were susceptible to the trauma due to the congenital malformation.
8 In Korea there was no report about Collet-Sicard syndrome caused by Jefferson fracture, although there were reports about it caused by meningioma and jugular vein thrombosis.
Although Connolly et al., reported a case about Collet-Sicard syndrome caused by Jefferson fracture, the assessment of paralysis of cranial nerve was dependent only on physical examination and laryngoscope.
10 In our case, however, nerve damage could be confirmed, and the degree of nerve damage and prognosis could also be determined through assessment methods such as VFSS and needle electromyography that objectively described the cranial nerve damage as well in addition to the physical examination.
In our case, decreased gag reflex in the left due to the damage of 9th cranial nerve was observed in the physical examination. The 10th cranial nerve damage was suspected based on the clinical dysphagia symptoms and electrodiagnostic abnormalities in the left cricopharyngeus muscle. In addition, VFSS showed findings of decreased laryngeal elevation and poor relaxation of upper esophageal sphincter due to paralysis of the vagus and glossopharyngeal nerve. Findings of atrophy of left trapezius muscle, winged scapula and fibrillation potentials/positive sharp waves in left upper trapezius muscle and sternocleidomastoideus in needle electromyography also enabled the diagnosis of paralysis of 11th cranial nerve. Moreover, deviation of the tongue to the left upon protrusion and increased insertional activity of left lingual root in the needle electromyography also provided findings that met the neuropathy in the 12th cranial nerve. No abnormal spontaneous activity was observed in lingual root in the needle electromyography, which was possibly due to the lower amount of damage of the 12th cranial nerve compared to that of other nerves. Consistent with the results of electromyography, deviation of the tongue on protrusion was recovered first clinically. Together with discussion on relevant literature, we report a case of Collet-Sicard syndrome that occurred after Jefferson fracture.