Aging of Skeletal Muscle Fibers
Article information
Abstract
Aging has become an important topic for scientific research because life expectancy and the number of men and women in older age groups have increased dramatically in the last century. This is true in most countries of the world including the Republic of Korea and the United States. From a rehabilitation perspective, the most important associated issue is a progressive decline in functional capacity and independence. Sarcopenia is partly responsible for this decline. Many changes underlying the loss of muscle mass and force-generating capacity of skeletal muscle can be understood at the cellular and molecular levels. Muscle size and architecture are both altered with advanced adult age. Further, changes in myofibers include impairments in several physiological domains including muscle fiber activation, excitation-contraction coupling, actin-myosin cross-bridge interaction, energy production, and repair and regeneration. A thorough understanding of these alterations can lead to the design of improved preventative and rehabilitative interventions, such as personalized exercise training programs.
INTRODUCTION
Aging demographics
By 2050, the world's population over 60 years will double from about 11% to 22% and there will be 2 billion people aged 60 or older living on this planet. Approximately 400 million will be 80 years or older [1]. Further, by 2050, 80% of older people will live in low- and middle-income countries. This increase in the number of people in older age groups is associated with an increase in life expectancy. For example, in 2012 in South Korea, life expectancy was 81.4 years (84.8 for women and 78.1 for men), which puts South Korea in 14th place among 182 countries in the world (Fig. 1). For comparison purposes it is worth noting that, during the same year, life expectancy in the United States was 78.7 years [2].
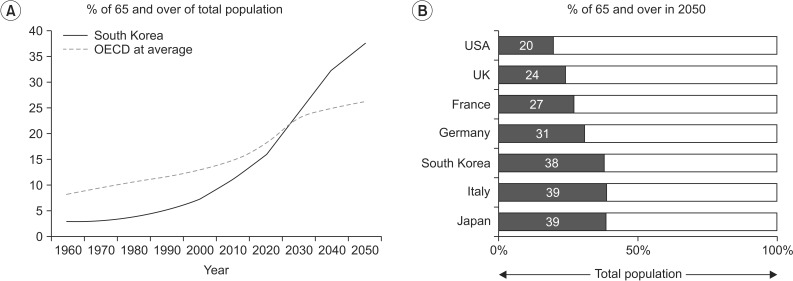
Percentage of old people in South Korea (A) in comparison with other developed countries (B) from 1960 to 2050 (projected). OECD, Organization for Economic Co-operation and Development.
The increase in the number of people in older age groups, per se, should not be considered a problem. However, aging is associated with an increased incidence of chronic health conditions and, perhaps more importantly, with an increase prevalence of impairment and disability. Visual and hearing impairments, cognitive decline, musculoskeletal disorders, frailty, and sarcopenia all reduce activity and restrict participation in personal, work-associated, and social activities. As a consequence, it has been estimated that, the number of older people requiring long-term care due to loss of functional independence will quadruple by 2050 [1].
Functional changes in elderly
Aging is associated with changes in body composition (increase in body fat and decreases in muscle and bone mass) which together with a decline in cognitive, visual, and hearing function, sleeping disorders, depression, and increased fatigue lead to a decline in physical function and significantly increases the risk for disability and loss of independence [3].
The prevalence of mobility limitations in elderly is high and is associated with frequent transitions between independent and dependent states. Several studies [4,5] have shown that older men and women transition more frequently from intermittent to continuous mobility limitation, than from no mobility limitation to intermittent mobility limitation. It is however possible to recover mobility after being disabled for 3 months (40% recovery rate) or even for 6 months (30% recovery rate). These findings suggest the road to disability in elderly is a dynamic process and offers several opportunities to restore function and recover independence [4,5,6,7].
Muscle strength is a strong predictor of severe mobility limitation, slow gait speed, increased fall risk, risk of hospitalization, and high mortality rate. For example, older adults with low muscle strength have a 2.6-fold greater risk of severe mobility limitation, 4.3-fold greater risk for slow gait speed, and 2.1-fold greater risk of mortality compared to older adults with high muscle strength [8]. The loss of muscle strength in elderly cannot be explained only by the characteristic presence of skeletal muscle atrophy. Several recent research studies show that other factors such as changes in central nervous system drive, peripheral nerve dysfunction, alterations in the neuromuscular junction structure and function, fat infiltration, and a number of complex cellular and molecular changes at the level of single muscle fibers impair muscle force generation and power production [9]. In this brief review we will summarize and discuss the cellular and molecular changes at the level of muscle fibers (Fig. 2) that contribute to the above.
DEFINING SARCOPENIA
One of the most distinctive characteristics of older people is the presence of skeletal muscle weakness and atrophy. The term sarcopenia was used for the first time by Rosenberg [10,11] to refer to the loss of lean body mass with aging. More recently, the European Working Group on Sarcopenia in Older People has expanded the definition and suggested criteria for diagnosis [12] including the presence of muscle weakness (for example handgrip strength), a lower muscle mass (determined by bioelectrical impedance or dual energy X-ray absorptiometry [DEXA]), and the presence of impaired performance (slow walking speed). The FNIH Sarcopenia Project Group [13] recently recommended normative values for muscle strength and lean body mass as follows: 1) grip strength in men <26 kg and in women <16 kg, or alternate grip strength adjusted for BMI <1.0 for men and <0.56 for women; and 2) appendicular lean body mass in men <19.75 kg and <15.02 kg in women, or appendicular lean body mass adjusted for BMI in men <0.789 and <0.512 in women.
The prevalence of sarcopenia in the older population may range from 4% to 27% depending on the gender of the participants and country. In Korea, the prevalence of sarcopenia has been reported to be 11.9% in women and 12.1% in men [14]. The loss of muscle mass and strength in older people is of greater magnitude in longitudinal compared to cross-sectional studies [15]. Further, more muscle mass and strength is lost by men compared to women particularly in muscles of the lower limbs [15,16]. In fact older women have relatively well-preserved strength in the muscles of the upper limbs. It must be noted that the loss of strength with advancing age is not a universal phenomenon and some individuals maintain muscle strength after 10 years of follow-up [17]. The explanation for this variability is not clear but it has been suggested that the level of physical activity is a contributing factor. In addition to the well-described loss of force sarcopenia is also associated with the loss of muscle power (force×velocity), particularly in older people with mobility limitations [18]. Power correlates very well with function and its loss is a good predictor of impaired mobility and falls.
SATELLITE CELLS
The function of satellite cells in normal skeletal muscle is to maintain the skeletal muscle homeostasis and enable skeletal muscle regeneration. These muscle-specific Pax7-expressing adult stem cells are normally quiescent, but when stimulated by damage or stress, they become activated and enter the cell cycle to either form new muscle fibers or self-renew and replenish the satellite cell pool, that will be used in the future [19,20,21].
At the level of the individual muscle fiber, sarcopenia may be associated with a reduced number of satellite cells; especially those associated with fibers expressing type II (fast) myosin heavy chain [22]. This is relevant because the majority of the motor unit and fibers lost with advanced adult age are type II. Moreover, the satellite cell activation in response to muscle damage is blunted in older adult men. This phenomenon is mediated by interleukin-6 which is thought to be a positive regulator of satellite cells proliferation and is transiently increased after acute exercise-induced trauma [23]. With aging, interleukin-6 becomes chronically elevated and promotes muscle catabolism most likely via suppressors of cytokine signaling proteins. These events reduce the efficacy of anabolic signaling pathways such as insulin-like growth factor 1 (IGF-1).
MUSCLE FIBER SIZE, STRENGTH, AND POWER
Normal adult muscle fiber size is reached between the ages 12 and 15 years. In normal muscle, there is less than 12% difference in the largest mean fiber diameter between all three muscle fiber types [24]. Both type I and type II (a and x) adult muscle fibers are larger in men than women. In men, type II muscle fibers are usually larger than type I fibers, whereas the opposite is true in women.
The decline in muscle mass, most prominent in the lower limbs, is accompanied by a 30% to 40% decrease in muscle fiber number between the second and eight decade [25]. Further evidence has suggested that apoptosis may play a considerable role in mediating the progression of muscle fiber loss in aging [26]. Mitochondrial-mediated apoptosis has been postulated as one of the mechanisms associated with muscle fiber loss. Muscle fiber size is also affected but to a lesser extent. The reduction in muscle fiber size is fiber type specific, with 10%-40% smaller type II fibers observed in muscle tissue collected from elderly compared with young controls [27]. In contrast, type I muscle fiber size is largely unaffected [22,28,29]. These fiber type specific changes can be explained by the age-related remodeling of motor units that result mostly in denervation of type II muscle fibers with collateral re-innervation of type I muscle fibers [30,31,32].
A reduction in whole muscle and individual fiber size is a contributor to sarcopenia but may not be the most important explanation for it. Changes in muscle fiber quality (force corrected for size) have been reported by several investigators [15,27,30,31]. In other words, loss of muscle strength and power with aging can in part be explained by reductions in intrinsic force-generating capacity of skeletal muscle fibers [32,33,34,35,36]. For example, Russ et al. [34] showed that older skeletal muscle exhibits a 34% reduction in its intrinsic force-generating capacity. The mechanism underlying this reduction includes age-related alterations in cellular and molecular processes such as changes in the satellite cell population, excitation-contraction coupling, myofilament interaction, mitochondrial function, and adipocyte infiltration [37,38] (see below).
Fiber type transformation
Muscle fiber type composition can change in response to various external stimuli in a fiber type-specific fashion. For example, type I fibers are more susceptible to inactivity and denervation-induced atrophy, while type II fibers are more affected with cancer, diabetes, chronic heart failure, and aging [39]. This difference in susceptibility can be explained by the activation and response to different signaling pathways. For example the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) protects type I fibers from atrophy while the transforming growth factor beta (TGFβ) family and the nuclear factor kappaB (NF-κB) predominantly affect type IIx fibers [39]. Aging is associated with a fast-to-slow fiber type shift, affecting mostly IIx fibers. As mentioned above, these muscle fiber changes are associated with age-dependent changes in motor unit composition [40].
Excitation-contraction coupling
Excitation-contraction coupling is a physiological process that converts the sarcolemmal action potential into muscle action and force generation. One of the key elements of this process are the dihydropyridine receptors located in the transverse tubule which are needed to activate calcium release from the sarcoplasmic reticulum through the ryanodine receptor. Once calcium is released into the myoplasm, it binds to troponin C and-through interactions with troponin I and T along with tropomyosin-results in actomyosin interaction. Calcium is then pumped back into the sarcoplasmic reticulum or competitively bound. Disruption or uncoupling at any step of this process may result in reduced muscle fiber activation, force generation, and lower whole muscle strength [41].
Aging causes a reduction in the number of dihydropyridine receptors, uncoupling between these receptors and the ryanodine receptors, and deficits in calcium release [37,38]. One mechanism to explain this sequence of events is the reduction in the expression of the sarcoplasmic reticulum junctional-face membrane (JP-45) [42] that alters the levels of specific dihydropyridine receptor subunits [38,42] interfering with the protein-protein interactions involved in excitation-contraction coupling [34].
Myofilament aging
The importance of age-related changes at the level of the myofilaments has been recently discussed [43]. The majority of studies demonstrate that older men and women have reduced single fiber maximal force even after adjustments for variability in fiber size. This is true in both type I and II fibers. Many molecular mechanisms have been proposed to explain such dysfunction including a reduction in myosin protein content; this may be particularly true in immobilized muscle [32]. The latter may be related to gene transcription with abnormalities in the myostatin gene and/or reduction in translation and protein synthesis leading to a lower myosin concentration per unit of muscle cell area. In addition, post-translational modifications of myosin via mechanisms such as oxidation and glycosylation may be present. Oxidative modification of myosin may disrupt the binding of the myosin head to the actin filament and, thus, reduce the number of actin-myosin cross-bridges in the strong-binding state [44]. This will limit force and power generation. Other than receptor changes, structural alterations of myosin causing a change in the kinetics of the cross-bridge cycle, has been suggested to contribute to age-associated muscle weakness [45]. It is interesting that longitudinal studies suggest that clusters of fibers that are not lost during aging try to compensate for these age-related differences in fiber size and quality [18,46,47] and although whole muscle performance is reduced, single muscle fiber properties may be relatively well preserved.
Other mechanical properties are also altered in older human. An increased in instantaneous stiffness (reduction in elasticity) has been reported in whole muscle as well as in single fibers [33]. This may be due to an increase in the number of cross-bridges in the weak-binding state but other factors such as changes in cross-bridge compliance and sarcomeric elements like titin may contribute. As previously noted, the role of this important protein in muscle actions has been recently reviewed [48]. In conclusion, independent of the mechanism(s) limiting normal myofilament interaction, substantial evidence supports the idea that muscle impairment in older people is, at least partially, a functional and not a quantitative problem.
Adipocyte infiltration
Aging is associated with increases in both intra- and inter-muscular adipose tissue and it has been showed that increased muscle fat content is associated with reduced muscle strength [49,50]. However, a direct relationship between increased intermuscular fat and age-related muscle weakness has not been established [51]. One of the potential mechanisms explaining how fat tissue decreases muscle force is the increased tumor necrosis factor alpha (TNF-α) production. It can be suggested that TNF-α may act directly on muscle fibers disrupting excitation-contraction coupling by altering intracellular calcium stores. This has been demonstrated to be the case in cardiac myocytes [52].
Mitochondrial function
Another example of the age-related alterations in muscle cell organelles is the loss of mitochondrial content and function [53]. Mitochondria respond to multitude of intracellular signals by modulating their function: ATP production, reactive oxygen species (ROS) production, and sensitivity to permeability transition. In aging muscle, the question is whether alterations in mitochondria represent a primary organelle defect versus a secondary (potentially adaptive) response to a changing cellular environment. Sensitization to permeability transition and release of mitochondrial-derived proapoptotic factors seems to be a constant finding in aging muscle regardless of the level of physical activity [54]. There are two possible mechanisms underlying these intrinsic mitochondrial changes: 1) mitochondrial genome damage that accumulates over the years and leads to impaired synthesis of mitochondria or synthesis of mitochondria with impaired function [55] and 2) disruption of mitochondrial turnover-mitochondrial removal and replacement-which results in accumulation of damaged mitochondria with impaired function [56]. Apart from this intrinsic alteration in mitochondrial function, extrinsic factors known as 'aging milieu' including oxidative stress and muscle fiber denervation may play a very important role in mitochondrial dysfunction [54]. It is interesting to note that the loss in mitochondrial function can be partially reversed by exercise training [57]. This is supported by the higher levels of oxidative enzyme and of molecular targets associated with mitochondrial biogenesis seen in endurance octogenarian athletes.
CONCLUSION
Clinical implications of muscle fiber aging
Sarcopenia is considered by many investigators, clinicians, and public health experts as an emerging threat because it leads to a loss of functional capacity, mobility, and independence. As a result, much research has focused on interventions to slow down or reverse sarcopenia in elderly. Abundant scientific evidence supports an important role for physical exercise in the prevention and rehabilitation of age-related functional decline and development of disability. However, a better understanding of the cellular changes associated with sarcopenia is needed before more specific interventions can be designed. In this brief review we have considered the evidence showing important intrinsic changes at the level of single muscle fibers that result in muscle weakness and atrophy in elderly.
In summary, age-related muscle changes are very complex and involve multiple features and mechanisms influenced both by intrinsic and extrinsic/environmental conditions. These muscle changes may look quite different than those associated with injuries, chronic diseases, and immobilization, so the therapeutic approach must be tailored to the individual case considering the changes at the muscle cell level. In order to prevent and treat sarcopenia effectively, future research is needed to elucidate where the targeted area at the muscle fiber level is for each individual.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
