Measurement of Knee Extensor Torque During Repetitive Peripheral Magnetic Stimulation: Comparison of the Forces Induced by Different Stimulators
Article information
Abstract
Objective
To investigate the factors that induce strong contractions during repetitive peripheral magnetic stimulation (rPMS) and compare the muscle torque induced by two stimulators (Stim A and Stim B) with different coil properties.
Methods
rPMS was applied to the right vastus lateralis of 30 healthy young adults. Stim A contained a 10.1 cm2 rectangular iron core coil, while Stim B contained a 191 cm2 round coil. The knee extensor torque (KET) induced by rPMS at 30 Hz was measured isometrically and divided by the maximum voluntary contraction (MVC) to obtain a relative value of MVC (%MVC). KET at 100% intensity of Stim A (A100%, 1.08 T) was compared to those at 100% or 70% intensity of Stim B (B100%, 1.47 T vs. B70%, 1.07 T). Additionally, we conducted a comprehensive literature search for studies that measured the KET during rPMS.
Results
Both the mean values of %MVC using B100% and B70% were significantly greater than that using A100%. Furthermore, the KET induced by Stim B was found to be larger than that described in previous reports, unless booster units were used to directly stimulate the main trunk of the femoral nerve.
Conclusion
Stim B induced a stronger muscle contraction force than Stim A did. This may be because the larger the coil area, the wider the area that can be stimulated. Additionally, a circular coil allows for deeper stimulation.
INTRODUCTION
Resistance exercises at 40%–85% of maximum voluntary contraction (MVC) has been reported to be important for preventing age-related muscle weakness and physical dysfunction [1-5]. However, resistance exercises are often difficult to continue performing because they depend on individual motivation. Therefore, electrical stimulation, which has little relevance to motivation, is used to maintain and improve muscle strength. Recently, application of repetitive peripheral magnetic stimulation (rPMS), which can be stimulated over clothing, to skeletal muscles has been reported to be useful for muscle strengthening as an alternative to electrical stimulation [6,7]. Since rPMS induces skeletal muscle contraction without activating cutaneous nociceptors [7], it produces less pain than electrical stimulation does [8,9].
Several rPMS intervention studies have reported muscle strengthening effects in healthy participants, patients with stroke and chronic obstructive pulmonary disease, and after total hip replacement [10-14]. However, in other studies, rPMS did not produce any significant changes in muscle thickness or cross-sectional area after 4 weeks [12] or did not clearly increase muscle strength in the lower extremities [15]. Thus, rPMS intervention studies have inconsistent results and are limited in number compared with studies on electrical stimulation. We have previously examined the stimulation methods, such the intensity and application site, and confirmed that strong muscle contraction can be obtained by selecting the optimal stimulation intensity and application site [16,17]. Differences in the shape of the stimulating coil can have a large effect on muscle contraction. Stimulation of the femoral nerve with a figure-eight coil reportedly induces greater muscle contraction than stimulation with a circular coil does [18]. Additionally, the larger the coil size, the stronger the muscle contraction force induced by femoral nerve stimulation, when the shape of the coil is the same [19].
Although the effects of equipment differences and stimulation methods on muscle contraction have been clarified gradually in our study and previous studies, reports on the measurement of rPMS-induced muscle contraction are limited. Recently, magnetic stimulators that can induce stronger muscle contractions without generating excessive heat have been developed; however, reports on the actual measurement of muscle contraction force using such stimulators are scarce. Thus, in this study, the muscle torques induced by two stimulators with different coil properties were compared, and the factors that caused strong contractions were examined. Furthermore, we conducted a comprehensive search for previous studies that measured the muscle contraction force during rPMS and compared our study results with those of the previous studies. The results of this study could aid in the clinical application of rPMS-induced muscle contractions for the prevention of disuse atrophy or strengthening of lower extremity muscle in older adults.
METHODS
Participants
Thirty healthy young adults (mean age, 21.5±4.1 years; 15 females and 15 males) without any history of orthopedic, neuromuscular, or central nervous system diseases voluntarily participated in this study. This study was conducted in accordance with the principles of the Declaration of Helsinki. The purpose of the study was explained in detail to all participants, both orally and in writing. Written informed consent was obtained from all the participants. This study was approved by the Ethics Committee of the Kawasaki University of Medical Welfare (No: 20-108; April 2021).
Equipment
Two types of magnetic stimulators manufactured by IFG Co. Ltd. (Stim A) and REMED Co. Ltd. (Stim B) were used for rPMS. Stim A and Stim B were air-cooled and oil-cooled magnetic stimulators, respectively (Fig. 1). Stim A contained a 10.1 cm2 rectangular iron core coil with rounded corners, while Stim B contained a large round coil (radius 7.8 cm, area 191 cm2). The maximum outputs of the magnetic stimulation just below the probe surface of Stim A and Stim B were 1.08 T and 1.47 T, respectively, at a frequency of 30 Hz.
Stimulation conditions
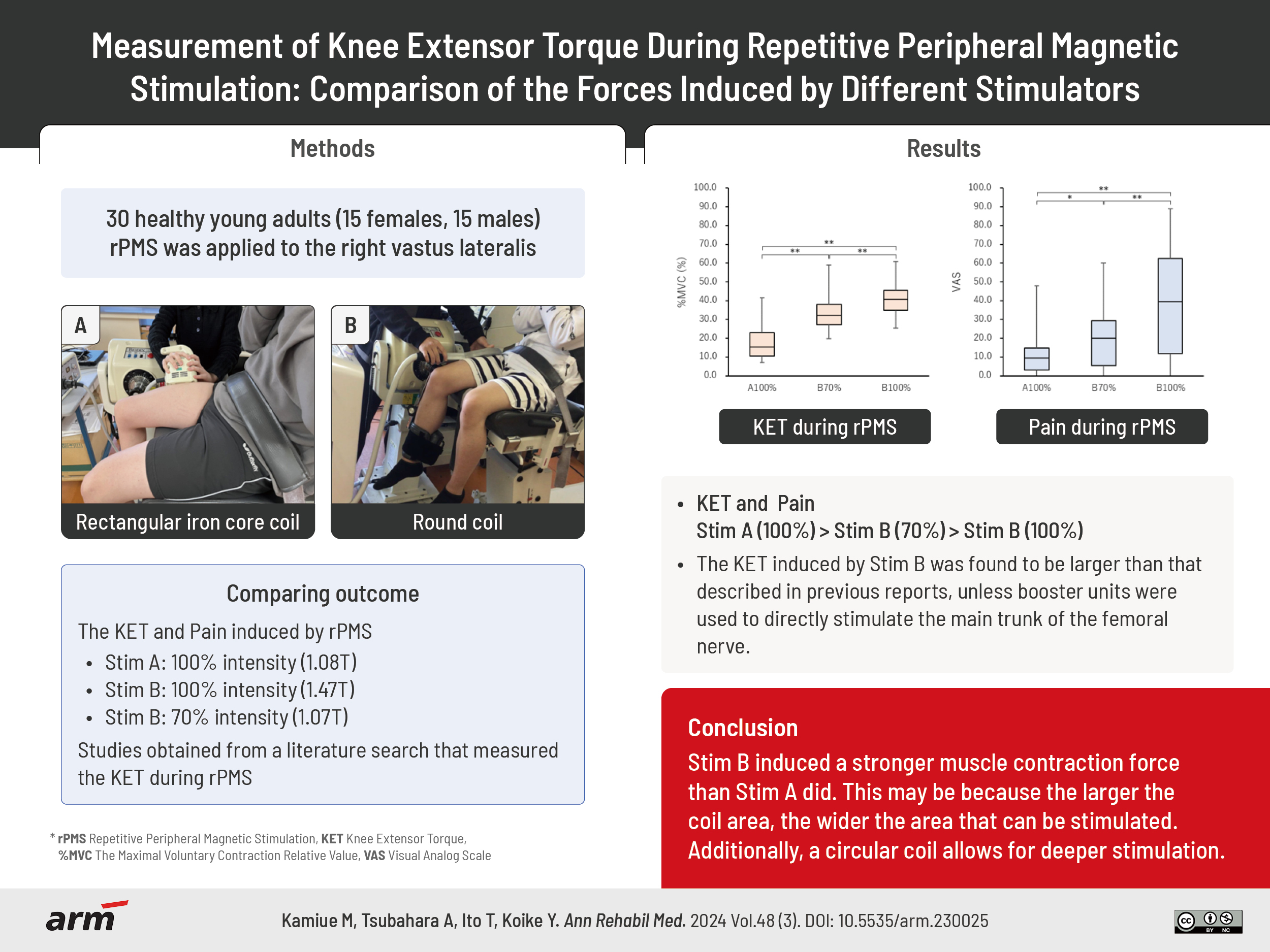
The right vastus lateralis (VL) was magnetically stimulated according to previous reports [17,20-23]. Participants were seated with their right knee and hip joints flexed at 75°. The trunk and pelvic girdle were strapped to the seat, and the distal end of the right lower extremity was strapped to a machine that measured the isometric contraction (Fig. 2).
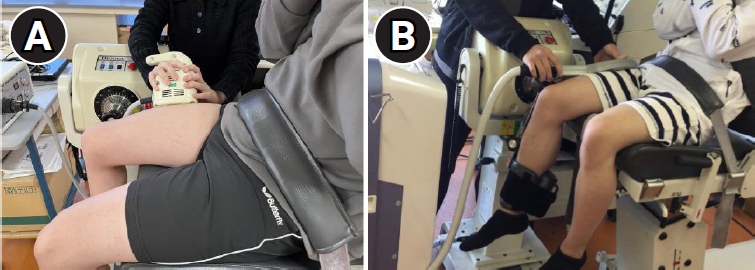
Muscle contraction induced by repetitive peripheral magnetic stimulation and measurement of muscle torque. (A) Contraction induced by the stimulator manufactured by IFG Co. Ltd. (Stim A). (B) Contraction induced by the stimulator manufactured by REMED Co. Ltd. (Stim B).
First, we used Stim A to identify stimulation points that could induce the maximum right VL contraction. The area between the proximal and distal 1/3 of the line connecting the anterior superior iliac spine and the superolateral margin of the patella was examined to identify the stimulation points [12]. Five to ten stimulations were applied to find the optimal point that could induce a stable and strong muscle contraction force; the identified point was marked.
When measuring the rPMS-induced muscle contraction force, great care was taken to ensure that the central part of the probe with the built-in coil coincided with the optimal stimulation point and that the long axis of the coil was parallel to the long axis of the thigh. Magnetic stimulation pulse trains were gradually increased to the maximum output intensity. After habituation to the magnetic stimulation was acquired, the VL was stimulated three times at the maximum output intensity of each stimulator (A100% and B100%). Each stimulation lasted 3 seconds with a 2 seconds rest interval between stimulations. Subsequently, additional muscle contractions were induced with Stim B at 70% of the maximum output intensity (B70%), that is 1.07 T/sec, to produce a magnetic stimulation output equivalent to that of Stim A at A100%.
The participants were instructed to relax their entire body as much as possible to avoid voluntary contraction of the quadriceps femoris during rPMS. The isometric knee extensor torque (KET) was measured three times in each condition (A100%, B100%, and B70%). Immediately after each stimulation, the degree of pain during rPMS was evaluated using a visual analogue scale (VAS). The participants were instructed to place an “x” mark on a 100 mm straight line. The left end (0 mm) of the line was set as “no pain” and the right end (100 mm) as “pain too intense to be tolerated.” The VAS values were recorded to one decimal place.
Measurement of KET
The right-sided KET was measured using a dynamometer (BIODEX System 3®; Biodex Medical Systems, Inc.), at a frequency of 100 Hz. Before MVC measurement, four sets of 3 seconds isometric knee extension exercises were performed with approximately 90% MVC as a warm-up. A rest interval of 5 seconds was maintained between the sets. After a 5 minutes post-warm up break, the muscle torque during a MVC of 3 seconds was measured twice with a 5 seconds rest interval between measurements. The larger of the two measurements was used in the analysis.
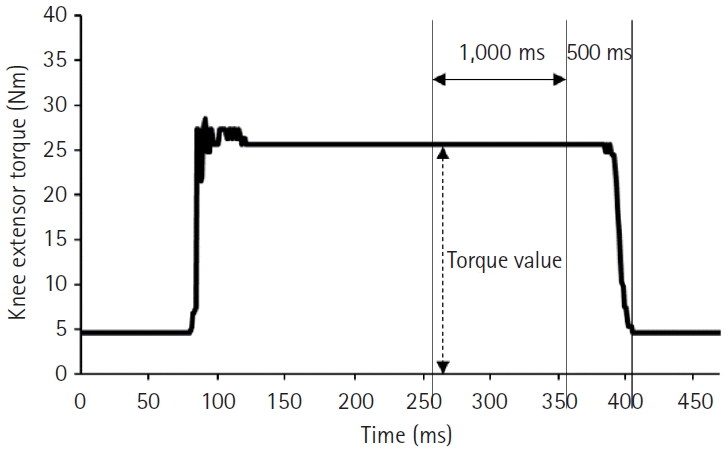
After another 5 minutes break, the rPMS-induced KET was measured at A100%, B100%, and B70%. The mean torque value for 1 second from 500 to 1,500 ms before the waveform returned to baseline after contraction was used for the analysis (Fig. 3). To compare the rPMS-induced muscle torque under the three conditions, the mean of the three measurements in each condition was calculated. The relative value of MVC (%MVC) was obtained by dividing the rPMS-induced KET by the MVC.
Data analysis
The Shapiro–Wilk test was performed on all data obtained in this study to determine if they were normally distributed. To examine the effects of the stimulation conditions on the KET (%MVC) and pain level (VAS value), their mean values at A100%, B100%, and B70% were compared. The Friedman test was used for multiple comparisons, followed by the Bonferroni correction for post-hoc testing. IBM SPSS version 22.0 (IBM Corp.) was used for the statistical analysis, and the significance level was set at p<0.05.
Comprehensive literature search
A comprehensive literature search was conducted to compare our study’s rPMS-induced KET with those of previous studies. PubMed was searched independently by two researchers. The titles, abstracts, and texts of studies published on rPMS in humans were searched without restrictions on publication date or methodological design.
First, an OR search was performed using “repetitive peripheral magnetic stimul*,” “rPMS,” “spinal magnetic stimul*,” “magnetic nerve stimul*,” “magnetic stimulation,” and “electromagnetic stimulation.” In the next OR search, the keywords used were “torque,” “force,” and “Nm.” In the third OR search, the keywords used were “quadriceps,” “vastus lateralis,” and “rectus femoris.” An AND search was then performed on the results of the three OR searches. The papers identified in the search were reviewed, and studies that actually measured the KET were selected. In addition, we manually searched for relevant studies among the references of the selected articles.
RESULTS
Comparison of the KETs in each stimulation condition
The Friedman test showed a statistically significant difference in %MVC between the different intensities (p<0.001). The mean MVC was 161.0±51.4 Nm (Table 1). The mean KETs induced by A100%, B100%, and B70% were 29.6±18.1 Nm (%MVC, 17.7%±8.3%), 66.1±24.5 Nm (%MVC, 41.1%±9.0%), and 54.9±23.4 Nm (%MVC, 33.8%±9.8%), respectively (Table 1). The mean %MVC at B100% was significantly greater than that at A70% and A100% (p<0.001). The mean %MVC at B70% was also significantly greater than that at A100% (p<0.001) (Fig. 4).
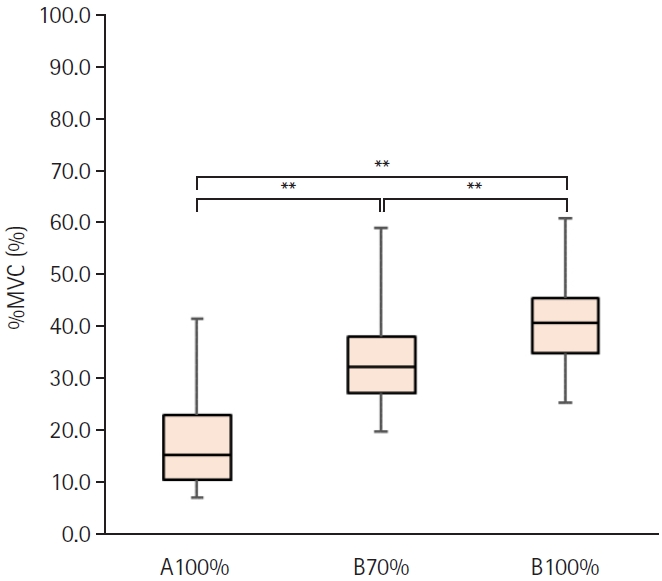
Comparison of relative value of maximum voluntary contraction (%MVC) during repetitive peripheral magnetic stimulation between each stimulation condition. %MVC was calculated by dividing the knee extensor torque during stimulation by the MVC. A100%, maximum output intensity of the stimulator manufactured by IFG Co. Ltd. (Stim A); B70%, 70% of maximum output intensity of the stimulator manufactured by REMED Co. Ltd. (Stim B); B100%, maximum output intensity of the Stim B. **p<0.01.
Comparison of the pain scores in each stimulation condition
The degree of pain evaluated using the VAS is shown in Fig. 5. The Friedman test showed a statistically significant difference in the VAS scores between the different intensities (p<0.001). The mean VAS score caused by A100% was lower than that caused by B70% (p<0.05) and B100% (p<0.001). The mean VAS score caused by B70% was also significantly lower than that caused by B100% (p<0.01). However, the pain caused by A100% was not uncomfortable.
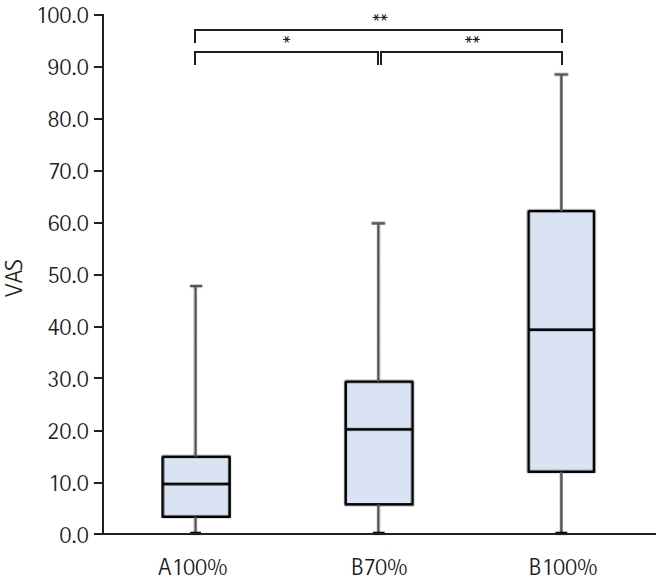
Comparison of pain during repetitive peripheral magnetic stimulation in each stimulation condition. A100%, maximum output intensity of the stimulator manufactured by IFG Co. Ltd. (Stim A); B70%, 70% of maximum output intensity of the stimulator manufactured by REMED Co. Ltd. (Stim B); B100%, maximum output intensity of the Stim B; VAS, visual analogue scale. *p<0.05, **p<0.01.
Comprehensive literature search
The AND and OR searches identified 115 papers published prior to October 2022. However, a review of the selected papers revealed that only four studies actually measured the rPMS-induced KET in the quadriceps femoris muscle. The magnetic stimulator used for transcranial magnetic stimulation (TMS), with stimulation intensities ranging from 1.8 to 2.0 T, was utilized in all four papers.
In a previous study in which the rectus femoris was stimulated, the maximum rPMS-induced KET did not exceed 20 Nm. In three other studies, in which the main trunk of the femoral nerve was directly stimulated with a stimulator equipped with booster units, the KET exceeded 20 Nm (Table 2). Among these three studies, in one study, the mean KET which was measured as the sum of four muscles, was greater than that of one muscle in our study. The other two studies using booster units reported a %MVC greater than 70%; however, the actual MVC values were not included in these papers.
None of the three studies that directly stimulated the main trunk of the femoral nerve reported on pain assessments. The study in which the rectus femoris was stimulated reported VAS scores of zero in all participants.
DISCCUSION
The results of this study revealed that the rPMS-induced KET with Stim B was greater than that induced by Stim A, even when the output intensity of the magnetic stimulation just below the probe surface was adjusted to be equivalent. The output intensity was previously measured, and B70% was found to be equivalent to A100%. The muscle contraction force induced by A100% was weaker than that induced by B70%, so we did not measure the muscle contraction force induced by A70%. Although the rPMS-induced contraction force is related to the magnetic stimulation intensity [15,16,18,19], coil size [19], and coil shape [18], no studies have compared the contraction forces induced by different coils at the same stimulation intensity. In the present study, the KET was compared by equalizing the stimulation intensities of the two different stimulators. Our study results indicate that not only the stimulation intensity, but also the size and shape of the coil affect the larger KET induced by Stim B than that induced by Stim A. The coil size of Stim B (191 cm2) was larger than that of Stim A (10.1 cm2). Although the stimulation was applied at the center of the optimal point, a fairly wide area of VL could have been stimulated by Stim B because it has a larger coil. The optimal point for magnetic stimulation in this study was just above the superficial proximal sub-branch of the VL branch arising from the femoral nerve trunk [17]. The central and distal sub-branches of the VL branch may also have been stimulated by Stim B. In addition, a part of the large coil was also placed on the rectus femoris; therefore, it may have cause partial contraction of the rectus femoris.
The coil shape in Stim A was rectangular with rounded corners, while that in Stim B was round. The round coil reportedly provides deeper stimulation than the rectangular coil [18]. Various factors influence muscle contraction force, and the cross-sectional area and volume of the muscle are known predictors of KET [24]. A thicker muscle reportedly produces a stronger KET [25]. Furthermore, thick subcutaneous fat decreases rPMS-induced KET because of the increase in electrical resistance and increase in the distance from the skin to muscles and nerves [20]. The present study results suggest that the deep proximal sub-branch of the VL branch was stimulated by the rounded coil of the Stim B by penetration of the magnetic waves through the subcutaneous fat. We hypothesize that Stim B was able to induce a stronger KET than Stim A, owing to the several factors mentioned above.
The intensity of resistance exercise commonly used to strengthen the quadriceps femoris muscle in older adults is reportedly 40%–85% of the MVC when performed 1–3 times a week for 6–52 weeks [1]. The present study revealed that stimulating the VL at B70% could induce more than 30% of the MVC in the knee extensors. The cross-sectional area of the VL is approximately 36.3% of the entire quadriceps femoris and that of the rectus femoris was approximately 11.4%; therefore, the contractile force of the VL or rectus femoris alone is approximately 1/3 or 1/9 [26]. Thus, when stimulated at B70%, >40% of the MVC of the VL alone can be induced, even if both the VL and rectus femoris contract. This value corresponds to the intensity of resistance exercises generally used for muscle strengthening. However, it was assumed that less than 40% of the MVC of the VL alone was induced when stimulated with Stim A. This contraction force may not have reached the intensity of resistance exercises required for muscle strengthening. Therefore, differences in the size and shape of the coils were found to be clinically important.
In the present study, the VAS value for Stim A was less than that for Stim B, and the higher stimulation intensity of Stim B produced a stronger pain. The relationship between intensity and pain in our study was consistent with those of several previous reports, validating the results of previous studies [7,8,21,27]. The VAS score for Stim A, with a smaller area, was less than that for Stim B. A large coil area expands the area being stimulated; thus, we hypothesized that Stim B was more likely to stimulate the Aδ fibers that conduct pain sensation than Stim A [28]. Therefore, stimulations with VAS values of less than 70/100 are clinically acceptable.
In this study, we compared the contraction force of a single muscle, namely the VL, between two stimulators when induced with rPMS. However, if rPMS were to be used for the purpose of strengthening muscles in a clinical setting, it would be necessary to stimulate various muscles. Additionally, even if the contractile force of stimulated muscles increases, it is unclear whether this contributes to physical activity. In the near future, intervention studies should be conducted to confirm that rPMS can be used on several muscles of the lower extremities in older persons to strengthen the muscles and to improve motor function.
The comprehensive literature search revealed that KET was measured in only four studies. In the study in which rPMS was applied to the rectus femoris using a stimulator without booster units, a strong contractile force was not induced. In the three other studies, although it was determined that >70% of MVC can be induced by direct stimulation of the main trunk of the femoral nerve, pain assessment was not reported. Because the femoral nerve contains sensory fibers, the participants must have experienced severe pain. Additionally, the stimulator was equipped with booster units to increase the stimulation frequency. However, considering the weight of the booster units, the clinical utility would be reduced [29-31]. The mean KET in the present study exceeded that of a previous report that stimulated muscle branches when induced at B100% (66.1 Nm) and B70% (54.9 Nm). Additionally, pain assessment indicated that Stim B was suitable for clinical use.
Bustamante et al. [10,11] reported on the effect of rPMS in patients with chronic obstructive pulmonary disease. Although KET induced by rPMS to the femoral nerve with a stimulator used for TMS was as low as 10 Nm, the MVC and walking endurance increased after 8 weeks of intervention [11]. However, stimulators used for TMS generate excessive heat with prolonged use. Therefore, to conduct intervention studies confirming the effect of muscle strengthening by inducing a strong KET, it is necessary to use a stimulator that does not generate excessive heat. These dedicated stimulators are expected to strengthen the muscles and improve walking endurance in older adults, but is problematic to conclude that they are a compensatory method for resistance exercise. Future studies are needed to examine whether rPMS improves physical function, whether there is pain or fatigue after continuous application of rPMS, and whether there is an overall feeling of satisfaction with rPMS.
Another limitation of this study is that the relationship between the participant characteristics and the rPMS-induced KET was not evaluated in detail. Various variables such as body size, BMI, thigh circumference, femoral length, and gender should be considered as factors of the muscle contraction force induced by rPMS. In the future, it will be necessary to investigate how the patient characteristics affect rPMS-induced KET. Based on these findings, the effectiveness of rPMS for muscle strengthening should be verified.
Notes
CONFLICTS OF INTEREST
Our university has signed a joint research agreement with OG Wellness Technologies Co., Ltd. The funders did not have any role in the study design, collection and analysis of data, preparation of the manuscript or the decision to submit the manuscript for publication.
FUNDING INFORMATION
We have borrowed equipment and received research funds from OG Wellness Technologies Co., Ltd.
AUTHOR CONTRIBUTION
Conceptualization: Kamiue M, Tsubahara A, Ito T. Methodology: Kamiue M, Tsubahara A, Ito T. Formal analysis: Kamiue M, Tsubahara A, Ito T, Koike Y. Funding acquisition: Kamiue M, Tsubahara A, Ito T. Project administration: Kamiue M, Tsubahara A, Ito T. Visualization: Kamiue M, Tsubahara A, Ito T. Writing – original draft: Kamiue M, Tsubahara A, Ito T. Writing – review and editing: Kamiue M, Tsubahara A, Ito T. Approval of final manuscript: all authors.
Acknowledgements
Our university has entered into a joint research agreement with OG Wellness Technologies Co., Ltd., from whom we have borrowed equipment and received research funds. We express our deepest gratitude to Dr. Hiroshi Ishida and Dr. Chiharu Kurozumi for their guidance and support.