- Search
| Ann Rehabil Med > Volume 47(6); 2023 > Article |
|
Abstract
Objective
To evaluate the efficacy of light-emitting diode (LED) and their dual-wavelengths as a treatment strategy for osteoarthritis.
Methods
We induced osteoarthritis in male Sprague-Dawley rats by intra-articular injection of sodium iodoacetate into the right rear knee joint. The animals with lesions were divided into an untreated group and an LED-treated group (n=7 each). In the LED-treated group, the lesioned knee was irradiated with lasers (850 and 940 nm) and dose (3.15 J/cm2) for 20 minutes per session, twice a week for 4 weeks. Knee joint tissues were stained and scanned using an in vivo micro-computed tomography (CT) scanner. Serum interleukin (IL)-6 and IL-18 levels were determined using enzyme-linked immuno-sorbent assay. Several functional tests (lines crossed, rotational movement, rearing, and latency to remain rotating rod) were performed 24 hours before LED treatment and at 7, 14, 21, and 28 days after treatment.
Results
LED-treated rats showed improved locomotor function and suppressed matrix-degrading cytokines. Micro-CT images indicated that LED therapy had a preserving effect on cartilage and cortical bone.
Conclusion
LED treatment using wavelengths of 850 and 940 nm resulted in significant functional, anatomical, and histologic improvements without adverse events in a rat model. Further research is required to determine the optimal wavelength, duration, and combination method, which will maximize treatment effectiveness.
Osteoarthritis (OA) is characterized by the loss of degraded articular cartilage, subchondral bone remodeling, osteophyte formation from hypertrophic bone changes, chronic inflammation including synovial membrane, and normal joint function [1]. The prevalence of symptomatic knee OA varies across countries, but overall, it ranges from 7% to 17% among individuals aged 40 years and older. OA is the fourth leading cause of disability globally, and contributes to medical and indirect costs due to job loss and early retirement [2].
Treatment options for OA can be broadly categorized into four main groups: (1) non-pharmacologic, (2) pharmacologic, (3) complementary and alternative, and (4) surgical. In general, the least invasive and safest treatment should be tried first before more invasive and expensive therapies are considered [3]. Pharmacologic treatments have the following problems. Patients taking nonsteroidal anti-inflammatory drugs should be aware of potential side effects, including complications related to cardiovascular system, kidney, and gastrointestinal bleeding [4]. And opioids have several problems such as cognitive impairment, delirium, and addiction. Intra-articular injections of corticosteroids, hydrogels, and other materials are another pharmacologic option for treating OA [3,5]. It is difficult to definitively determine which material is the most effective because different formulations have own advantages and disadvantages, and different efficacy [3,6]. While there is evidence supporting the use of these materials to reduce functional limitations, there is a lack of comparison with a placebo or control group [3]. The most effective treatment option is total knee replacement for patients whose symptoms have not responded to other treatments. However, surgical treatment can lead to a variety of complications, including prosthesis infection, venous thromboembolic disease, neurovascular injury, and peri-implant fractures [7]. Even after successful surgery, about 25% of patients report pain and disability for more than a year, requiring additional rehabilitation and reoperation. Consequently, surgical treatment should be withheld as a last resort. These are why new non-pharmacologic treatment options are need to be researched.
Low-level laser therapy can trigger in vitro, in vivo, and in human photobiochemical reactions in multiple conditions of various tissues: neck muscle pain, nerve injury, soft tissue wound healing [8-12]. Light-emitting diodes (LEDs), which are composed of semiconductors, have the ability to convert electrical currents into narrow-spectrum light that its incoherent in nature. LEDs are widely used worldwide due to their relatively low price and high accessibility compared to laser. For this reason, treatment using LED is also being performed in various conditions: oral mucositis, hearing loss, temporomandibular disorder, and traumatic brain injury [10]. Treatments using light reduce pain and inflammation and act like opioids without the side effects of addiction, and toxicity. These treatments also act on tissues to promote cell proliferation, the healing process, and tissue regeneration, prevent cell death, and reduce pain and inflammation [13]. LED can also trigger in natural intracellular photobiochemical reactions [14].
However, while laser treatment equipment is expensive and has limitations in approach, there have been limited studies examining the effects of LED, and the results reported have been inconsistent. More recently, several chromophores have been studied in relation to the mechanisms by which they produce therapeutic effects on responses called photobiomodulation [15]. Among them, studies have been conducted focused on cytochrome c, an oxidase electron transport system located in the inner mitochondrial membrane that absorbs infrared light in the 760–940 nm region [16]. Inflammatory responses and oxidative stresses were reduced in burn wounds after 850 nm wavelength LED treatment, which promotes wound healing [17]. It has the effect of reducing pro-inflammatory cytokines in mice with colitis after 940 nm wavelength LED treatment [18]. Prior study highlighted that combining LED wavelengths is more effective than using a single wavelength alone in skin lesion [19]. Therefore, the purpose of this study was to determine whether the combination of the 850 nm, and 940 nm wavelengths are effective for OA.
All animal experiments and surgical procedures conducted in this study were approved by the own Institutional Animal Care and Use Committee (IACUC) of Yonsei University Wonju College of Medicine with an identification code of YWC-211014-1. The procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Adult male Sprague–Dawley rats (250 g) were obtained and housed under a 12 hours light/12 hours dark cycle at a constant room temperature (20°C–22°C), with free access to food and water. Male Sprague-Dawley rats were anesthetized with a mixture of 3% isoflurane, 80% nitrous oxide (N2O), and 20% oxygen (O2). To maintain a constant body temperature of 37°C during the surgical procedure, heating pads were used.
As shown in Fig. 1, for the induction of OA, monosodium iodoacetate (MIA, 30 mg; Sigma-Aldrich) dissolved in 50 pL of sterile physiological saline solution was injected through the infrapatellar ligament of the right knee joint on day 0 [20]. Seven days after injection, animals that demonstrated gait disturbance during horizontal ladder walking were considered successfully lesioned and used for further experiments. The lesioned animals were divided into two groups. Animals were sacrificed at four weeks after LED treatment. The internal control for this study involved using the contralateral (left) leg as a reference.
The high-density (HD) optical probe and high-power (HP) LED system comprised a HP-LED module, a HD optical probe, and a system controller. The HP-LED module comprised two HP-LED units and two waveguide units. Light outputs were transmitted, mixed through the waveguides, and fed into two custom-made HD optical probes. Each probe, with a diameter of 1.3 mm and a length of 500 mm, consisted of 500 multi-component glass fibers, which are suitable for medical applications. The core and cladding had diameters of ~45 µm and ~50 µm, respectively, and the probes were coated and encased in a jacket to protect against mechanical and environmental stresses. Various operational parameters, including the system on/off switch, light output power, irradiation time, and data record, were processed by the system controller with a micro controller unit and an HP-LED driver. The emission wavelengths were 850±20 and 940±20 nm in a 25 mW LED (PT-100; M.I.One). All LED ran intense pulsed light at 100 Hz (duty ratio=21%). The mean energy per unit of the optical fiber was 25 mW for both the 850 and 940 nm LED.
The rats were anesthetized and fixed in an acrylic holder, and the LED probe was attached to the right knee joint (Supplementary Fig. S1). LED stimulation was performed in a 0.5 cm2 area with the following parameter settings: 20 minutes per session, twice a week for 4 weeks, and LED intensities at 6.25 mW of power for each wave length (850 and 940 nm), which is 25% of the 25 mW machine output [20-23]. The stimulation was then 12.6 mW/cm2 for each wave length with 21% of duty ratio in 0.5 cm2 area. The daily energy dose applied is 3.15 J/cm2. The control group did not receive LED irradiation under the same conditions as the experimental group.
Functional tests were conducted on all animals at various time points: 24 hours prior to treatment and at 7, 14, 21, and 28 days following LED treatment. Prior to the onset of OA, all rats exhibited normal behavior and were capable of performing the tests. Trained and experienced observers, who were unaware of the treatment groups, administered and scored all behavioral tests. Changes in body weight were determined by subtracting the baseline weight from the weight measured after the injection of MIA. Motor function in rats with OA was evaluated using an open field test and rotarod test. The open field test involved placing the rats in an acrylic box divided into 12 squares (10×10 cm each) with black walls and a white floor. The animals were positioned in the central area of the open field arena and assessed in a quiet room environment. The test consists of placing an animal in an open field arena marked by a grid or lines, and recording the number of times the animal crosses the lines.
The number of lines crossed in line-crossing test (defined as the presence in a quadrant of at least two paws and the nose), amount of rotational movement (unidirectional circling), and amount of rearing (animal standing upright on its hind legs) were recorded for 2 minutes [24-26].
A rotarod apparatus (Panlab rotameter; DL Naturegene Life Sciences, Inc.) was used to assess motor performance. The performance of the animals was assessed by measuring the time it took for them to remain on a levitated rotating rod, which had a diameter of 3 cm. Prior to the actual testing, three acclimation trials were conducted at a constant speed of 5 rpm, each lasting for 3 minutes. To establish a baseline performance, a pretraining session on the rotarod was conducted for three consecutive days, with three trials per day. During the pretraining, the speed of the rod accelerated linearly from 4 to 100 rpm. Each trial had a maximum duration of 5 minutes, with a 3-minute rest interval between trials to prevent fatigue. During each trial, the latency to fall from the rotating rod was measured. If the animal fell, it was promptly placed back on the rod, and this process could be repeated up to 5 times in a single session. The latency to falling was automatically recorded using photo-cells, and the cumulative latencies on the rod for each day were analyzed. If the animal completed three full revolutions while spinning on the rod, the trial was terminated. Testing was conducted on days 0, 7, 14, 21, and 28.
The collected samples were fixed in 8% formaldehyde and subjected to scanning using a Skyscan 1176 in vivo micro-computed tomography (CT) scanner (Bruker Micro-CT). The scanning parameters included a voltage of 65 kV and a current of 278 μA. A total of 360 views were obtained at 0.5° angle increments, with each view having an exposure time of 520 ms. This scanning process resulted in a resolution of 18 μm. The raw data obtained from the tibiae were processed using NRecon software (Bruker Micro-CT) to generate two-dimensional grayscale image slices. Subsequently, a CT analyzer (CT-AN, v1.10.9.0; Bruker Micro-CT) was utilized to assess various structural parameters of the subchondral bone, such as bone volume fraction (BVF), mean polar moment of inertia (MPM), cross-sectional thickness (CST), and bone mineral density (BMD).
The rats underwent transcardial perfusion, in which 100 mL of 0.9% sodium chloride solution was initially infused, followed by 200 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). By carefully removing the leg muscles, the knee joint, containing parts of the femur and tibia, was removed. Tissues were fixed for 12 hours, decalcified, and embedded in paraffin. Sagittal sections with a thickness of 4 μm were obtained using a microtome, and these sections were subsequently stained with hematoxylin-eosin-saffron and Safranin O-Fast Green.
For all measurements light photo micrographic images were acquired on a Nikon Optiphot microscope (Nikon Inc.) fitted with a Nikon digital camera (DXM1200), using Nikon ACT-1 image capture software (ver. 2.2). The images were imported into Adobe Photoshop (ver. 7.0, Adobe Systems Inc.) and were adjusted for brightness and contrast to optimize photographic representation of the images obtained by the microscope. Acquired cartilage images were divided three regions, anterior, middle and posterior then articular cartilage thickness was measured at the dividing lines between anterior/middle, and middle/posterior regions [27]. It is presented as the average of the anterior and posterior articular cartilage thickness.
At the time of sacrifice, serum was collected from each rat. Serum was spun down, aliquoted, and stored at -80°C until use. The enzyme-linked immunosorbent assay (ELISA; R&D Systems) and commercial kits (MBL-Medical & Biological Laboratories) were used to measure serum levels of interleukin (IL)-6 and IL-18, respectively. A plate reader (Bio-Rad) was used to measure the readings at a wavelength of 450 nm. These readings were compared to a standard curve created using known concentrations of the recombinant mediators. The detection limits for IL-6 and IL-18 were determined to be 0.09 pg/mL and 12.5 pg/mL, respectively.
Statistical analysis was conducted using Prism (GraphPad Software) for the t-test and two-way repeated ANOVA. Post-hoc comparisons were performed using the Bonferroni method. Data are presented as the mean±standard error of the mean. The significance level was set at p<0.05, unless stated otherwise.
To monitor the overall health status of the rats, we measured their body weight weekly for a period of 4 weeks following the LED treatment. Anxiety and depression levels were assessed using the open field test [28]. In the experimental groups receiving LED treatment, there was an approximate 10% reduction in body weight. However, there were no statistically significant differences (p=0.053) observed between the untreated OA group and the LED-treated OA group in terms of body weight (Fig. 2A).
We assessed locomotor function and weight bearing using the rotarod test and open-field test weekly. The number of line crossings of non-treated rats decreased throughout the treatment period (1.25±1.23), while that of LED-treated rats was maintained at 9.66±1.51 for four weeks post-treatment, implying that LED treatment facilitated motor improvements (Fig. 2B). To evaluate hind limb weight bearing, rearing was assessed. LED-treated rats reared significantly more (6.5±0.85, 3.0±0.91) than the untreated group (3.75±1.18, 0.75±0.25) at 7 and 28 days (Fig. 2C). In the rotarod test, the LED-treatment group exhibited significant increases in the time until falling compared to the untreated group. Specifically, at 7 days after LED treatment, the LED-treatment group showed a time until falling of 46.50±4.21 (mean±standard deviation), while the untreated group had a time until falling of 25.0±2.65. Similarly, at 28 days after LED treatment, the LED-treated group had a time until falling of 48.50±5.95, whereas the untreated group had a time until falling of 29.5±4.82 (Fig. 2D). The LED-treated group demonstrated an improvement in locomotor function compared to the untreated group at both 7 and 28 days after treatment (Fig. 2B-D).
To determine the protective effect of LED treatment against OA, we administered LED stimulation to rats 7 days after MIA intra-articular injection, and evaluated cartilage damage after 4 weeks of LED treatment. Safranin O–staining demonstrated proteoglycan loss after MIA injection (Fig. 3). The articular cartilage thickness of normal rat model was 123.0±6.22 μm. It was 41.18±3.00 μm in OA control rat model. Articular cartilage thickness was statistically significantly thicker in the LED-treated OA rat model (61.87±5.66 μm, p=0.004).
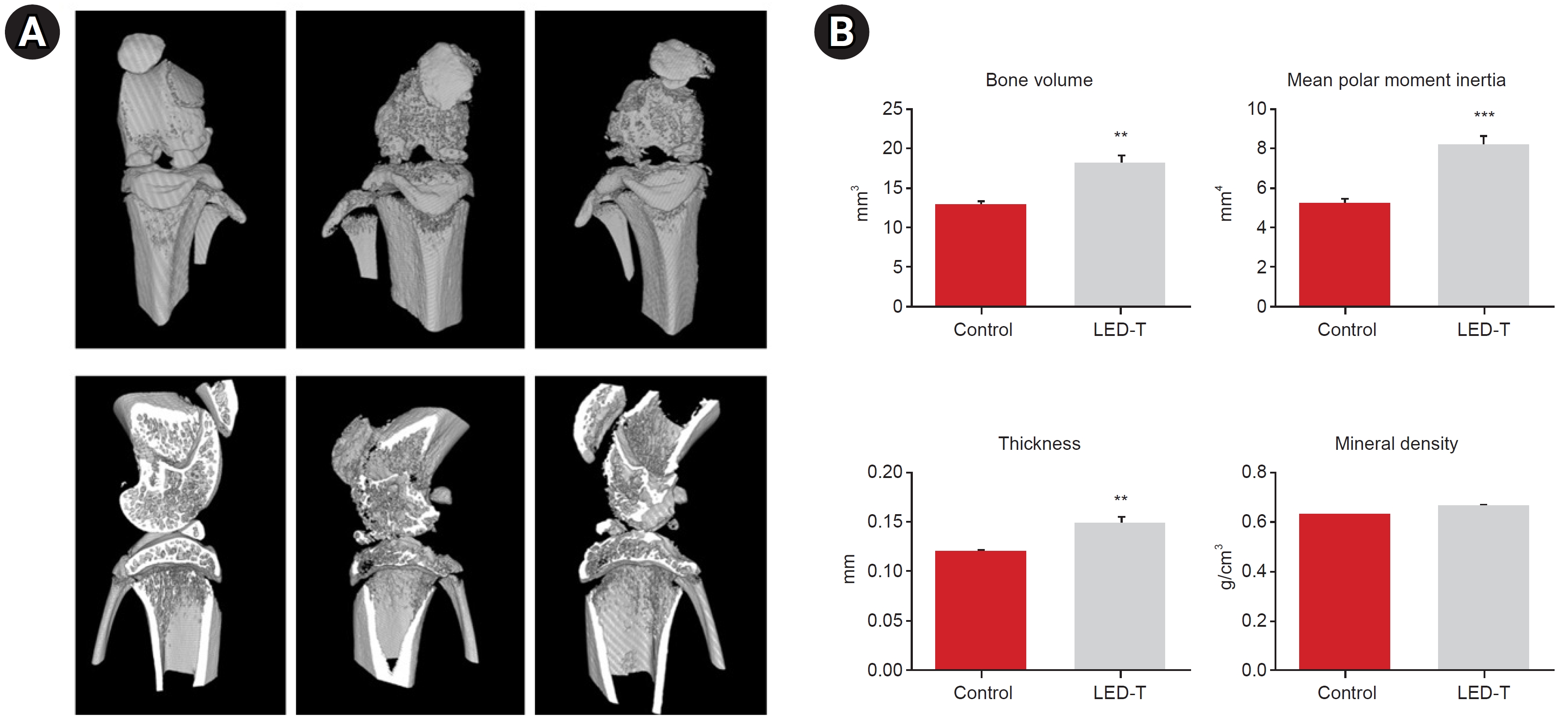
Micro-CT images indicated that LED therapy exerted a restorative effect on degenerated cartilage and cortical bone. In the LED-treated group, there was a significant increase in BVF and CST at both the medial and lateral sides compared to the untreated group. BVF represents the total bone volume, while CST reflects the thickness of the subchondral plate. Similarly, BMD and MPM, which represent bone strength and bone stiffness, respectively, also increased significantly in the LED treatment group (Fig. 4).
The levels of IL-6 and IL-18, pro-inflammatory cytokines associated with the degradation of articular cartilage, were quantified in the serum using an ELISA. The serum concentrations of IL-6 was significantly decreased in the LED-treated group (103.3±12.38 pg/mL) compared to the untreated group (259.5±47.74 pg/mL) after LED treatment (p=0.002; Fig. 5A). The serum concentrations of IL-18 also decreased in LED treatment group (123.6±13.11 pg/mL), compared to untreated group (218.8±26.97 pg/mL) after LED treatment, but this was not statistically significant (p=0.051; Fig. 5B). Although there was a difference in statistical significance, LED treatment tended to suppress IL-6 and IL-18, which are pro-inflammatory cytokines involved in OA [29].
The purpose of this study was to determine whether a combination of the following two wavelengths would be effective for OA: (1) 850 nm, which is the middle wavelength of the two studies (830 and 870 nm) that reported a therapeutic effect for OA, and (2) 940 nm, which is the 900 nm wavelength region that has reported stimulatory effects on osteogenesis and several other conditions such as RA, and colitis [18,20,22,30,31]. We referred to previous studies that examined the interactions between light and tissue, specifically considering the intensity and duration of exposure to light-based devices [15].
For LED-emitted photons to exert an impact on a living biological system, they need to be absorbed by a molecular chromophore or a photoacceptor. Within the mitochondria and cell membranes, there are various light-absorbing entities such as porphyrins, flavins, and other chromophores. When exposed to light of suitable wavelengths and doses, these chromophores absorb the light and initiate biological responses [15]. Increasing evidence suggests that the photobiomodulation mechanism result in the cellular reaction cascade involving the activation of mitochondrial respiratory chain components. Different wavelengths of light are absorbed by different chromophores and have different effects on tissues [15]. The wavelength with high therapeutic effect is different for each disease [32,33]. In addition, the possibility of resulting a synergistic effect by combining wavelengths of different lengths was also reported [22,34].
In this study, the LEDs treated OA rat models exhibited in functional: line crossing, rearing, and rotarod tests (Fig. 2), histological: cartilage thickness (Fig. 3), and anatomical: BVF, MPM, and CST (Fig. 4) improvements without visible side effects. The LED-treated group demonstrated higher values than the control group for three parameters, in addition, IL-6 and IL-18 serum concentrations significantly decreased (Fig. 5). In the safety evaluation, there were no abnormal findings, such as skin redness, burns, and edema.
Several similar studies evaluated whether LED irradiation could be effective as a non-invasive therapeutic strategy for the treatment of OA. In a particular study, OA was induced in the 24 rats by administering an intra-articular injection of 3 mg of MIA through the patellar ligament of the right knee. To assess the effect of LED irradiation, indomethacin was administered orally, 7 days after MIA injection. Radiographic examination did not reveal any differences between the indomethacin and LED treatment group [35]. In the histological analysis, however, the use of LED irradiation demonstrated a protective effect against cartilage damage and subchondral bone destruction. It also significantly reduced the infiltration of mononuclear inflammatory cells and the formation of pannus. In this study, authors used an LED wavelength of 840 nm, whereas we used a mixed wavelength of 850 and 940 nm. Both studies showed that LED irradiation may be an effective treatment for OA. However, only radiographic and histological analyses were analyzed in 840 nm wavelength study, whereas functional, anatomical, and histological studies were analyzed in this study.
Given the evidence indicating the diverse cellular effects of photobiomodulation, notably its anti-inflammatory properties, the combination of LED therapy with antioxidant treatment emerges as a novel approach for the prevention and treatment of early-stage OA. Wavelength of 635 nm was used to irradiate hydrogen peroxide induced OA like chondrocytes. Pre-treatment with 635 nm of LED light for 2 hours significantly reduced free radical formation (from 100% to 8.25%) and the expression of the inflammatory genes IL-1b and TNF-α in OA-like cells, while the expression of the matrix gene COL-2 was enhanced. Furthermore, a combination of preventative LED treatment with antioxidant treatment exerted synergistic anti-inflammatory effects and a reduction in MMP-13 expression [21]. Another study reported similar results with no significant differences in mRNA expression in cartilage, but there were increased type II collagen expression, and decreased TNF-α expression [20]. According to these study, preventive LED irradiation combined with antioxidant therapy is a novel therapeutic strategy for treating early-stage OA.
OA progression leads to articular cartilage degeneration which is characterized by decreased bone volume, bone density, bone stiffness, and thickness of subchondral bone [36-38]. To observe the morphological changes in subchondral bone, we obtained the parameters such as BVF, MPM, CST, and BMD derived from micro-CT analysis. All relevant parameters were significantly increased in the LED-treatment group. These results show that LED treatment restores the microstructure of subchondral bone and alleviates abnormal bone remodeling caused by osteoarthritis.
Our study had three main limitations. First, the number of rats used in this experiment was not sufficiently large. Second, this study only evaluated short-term therapeutic effects, and long-term therapeutic effects should be assessed in the future. Also, we could not evaluate the score of OA parameters, including articular cartilage destruction (OARSI grading system), because of the severe degradation of articular cartilage. Because different wavelengths can show different effects, it is important to precisely define the disease and condition. In pressure ulcer, 658 nm is more effective than 808 and 940 nm [33], whereas in oral ulcer, 810 nm is more effective than 940 nm [32]. In addition, although each of the wavelengths of 810 and 940 nm did not have a beneficial effect, it is necessary to confirm whether a combination of them has no effects [33]. One of the essential parts is to determine whether they are safe when applied to the human body, even though wavelengths and parameters are discovered that have therapeutic effects.
It is necessary to find the effect of the combination between LED wavelengths as well as between LED wavelengths and non-pharmacologic modality therapy, or between LED wavelengths and invasive procedure [39,40]. Furthermore, more studies should be conducted to verify the efficacy of these treatments in the parameter settings of the device and characteristics of the altered stem cell and cartilage [39,40]. Determining optimal treatment parameters and safety margins is also necessary, as cellular activity depends on wavelength, power density, and irradiation time.
In conclusion, combination of 850 and 940 nm wavelengths LED therapy produced functional, anatomical, and histological improvements without side effects in a rat model of OA.
FUNDING INFORMATION
This research was supported by a grant of regional specialized industry development R&D Project through the KIAT (Korea Institute for Advancement of Technology), funded by the Ministry of SMEs and Startups, Republic of Korea (S3090288).
AUTHOR CONTRIBUTION
Conceptualization: Choi WW, Kim SH, Lee JY, Yong SY. Methodology: Choi WW, Kim JH, Yong SY. Formal analysis: Kim HS, Lee H. Funding acquisition: Kim SH, Kim JH, Yong SY. Project administration: Choi WW, Yong SY. Visualization: Kim HS, Lee H, Lee JY. Writing – original draft: Choi WW, Kim K, Kim SJ, Kim M, Yong SY. Writing – review and editing: Choi WW, Kim SH, Yong SY. Approval of final whole manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5535/arm.23138.
Summary of current evidence of neurodevelopmental assessment tool
Supplementary Fig. S1.
The rats were anesthetized and fixed in an acrylic holder, and the light-emitting diode probe was attached to the right knee joint.
Fig. 1.
Schematic overview of the experimental design. MIA, monosodium iodoacetate; LED, light-emitting diode.
Fig. 2.
Figure 2. Improvement of motor function after light-emitting diode (LED) light irradiation treatment in degenerative osteoarthritis model. (A) Change of body weight during LED treatment. LED treatment did not change the general condition including food uptake, depression, anxiety in monosodium iodoacetate-induced osteoarthritis rats. (B) Line cross test. (C) Rearing test. (D) Rotarod test. (B-D) LED treatment significantly increased hind limb motor function. The asterisk (*) denotes a statistically significant difference between un-treated (control) group and LED-treated (LED-T) group at the 0.05 level, **p<0.005, and ***p<0.0005.
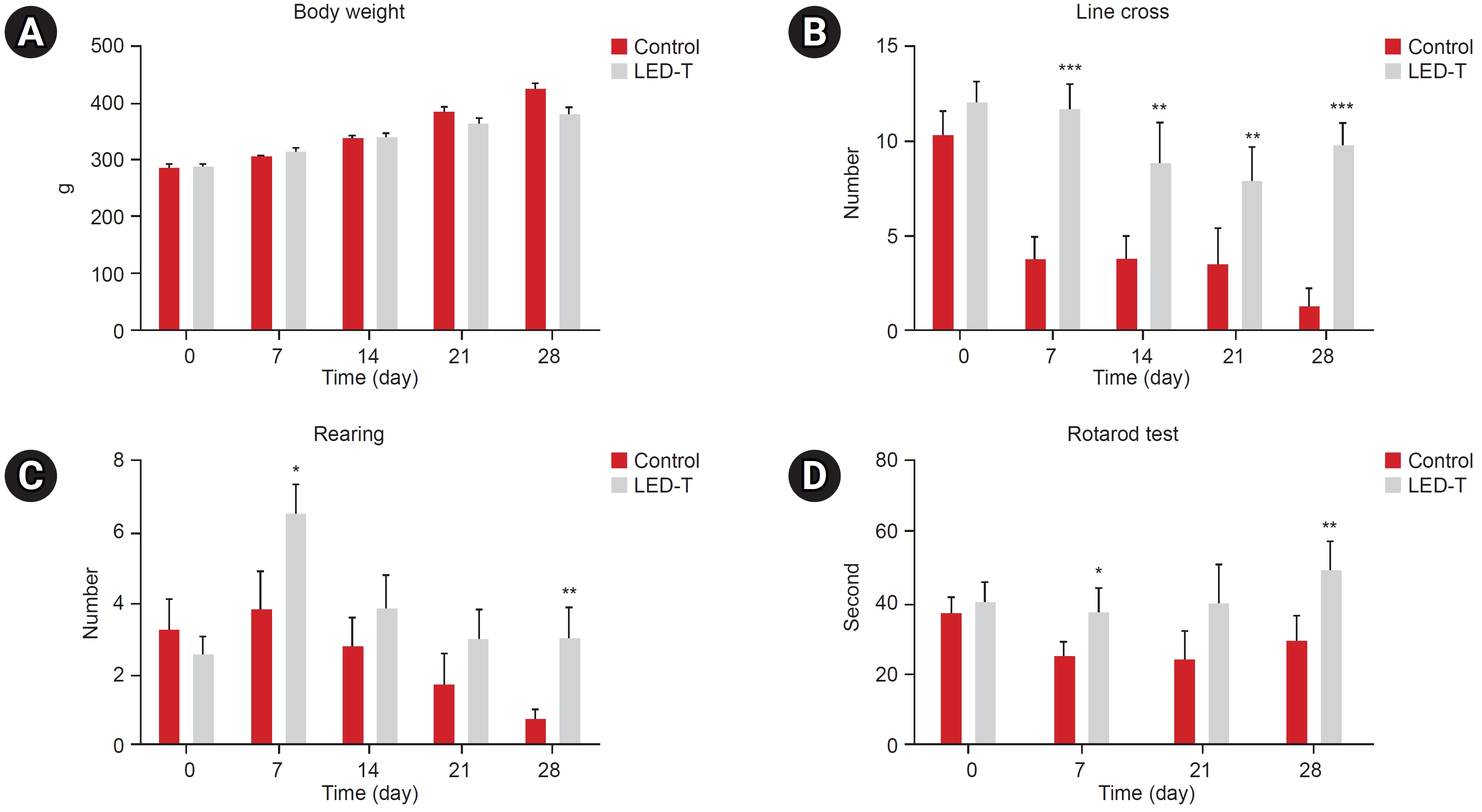
Fig. 3.
Articular cartilage thickness differentiation after light-emitting diode (LED) light irradiation treatment in degenerative osteoarthritis (OA) model. (A) Changes in articular cartilage thickness illustrated by hematoxylin and eosin and Safranin O-Fast Green staining after LED light irradiation treatment. Scale bar=50 μm. (B) Quantification of articular cartilage thickness. LED-T, LED-treated.
**p<0.005.

Fig. 4.
Micro-computed tomography (CT) images of degenerative changes in articular cartilage following light-emitting diode (LED) light irradiation treatment. (A) Anatomical change in CT image. (B) Analyzing of bone volume, mean polar moment inertia, cross sectional thickness and mineral density. LED-T, LED-treated.
**p<0.005, ***p<0.0005.
REFERENCES
1. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol 2008;22:351-84.


3. Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician 2012;85:49-56. Erratum in: Am Fam Physician 2012;86:893.
4. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 2020;180:114147.



5. Mou D, Yu Q, Zhang J, Zhou J, Li X, Zhuang W, et al. Intra-articular injection of chitosan-based supramolecular hydrogel for osteoarthritis treatment. Tissue Eng Regen Med 2021;18:113-25.




6. Rahimi M, Charmi G, Matyjaszewski K, Banquy X, Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater 2021;123:31-50.


7. Quinn RH, Murray JN, Pezold R, Sevarino KS. Surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg 2018;26:e191-3.


8. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009;374:1897-908. Erratum in: Lancet 2010;375:894.

9. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg 1971;122:532-5.


10. Mussttaf RA, Jenkins DFL, Jha AN. Assessing the impact of low level laser therapy (LLLT) on biological systems: a review. Int J Radiat Biol 2019;95:120-43.


11. Rochkind S, Geuna S, Shainberg A. Chapter 25: phototherapy in peripheral nerve injury: effects on muscle preservation and nerve regeneration. Int Rev Neurobiol 2009;87:445-64.


12. Tchanque-Fossuo CN, Ho D, Dahle SE, Koo E, Li CS, Isseroff RR, et al. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen 2016;24:418-26.



13. DE Oliveira MF, Johnson DS, Demchak T, Tomazoni SS, Leal-Junior EC. Low-intensity LASER and LED (photobiomodulation therapy) for pain control of the most common musculoskeletal conditions. Eur J Phys Rehabil Med 2022;58:282-9.

14. de Boer E, Warram JM, Hartmans E, Bremer PJ, Bijl B, Crane LM, et al. A standardized light-emitting diode device for photoimmunotherapy. J Nucl Med 2014;55:1893-8.


15. Barolet AC, Villarreal AM, Jfri A, Litvinov IV, Barolet D. Low-intensity visible and near-infrared light-induced cell signaling pathways in the skin: a comprehensive review. Photobiomodul Photomed Laser Surg 2023;41:147-66.


16. Hamblin MR, Liebert A. Photobiomodulation therapy mechanisms beyond cytochrome c oxidase. Photobiomodul Photomed Laser Surg 2022;40:75-7.

17. Silveira PC, Ferreira KB, da Rocha FR, Pieri BL, Pedroso GS, De Souza CT, et al. Effect of low-power laser (LPL) and light-emitting diode (LED) on inflammatory response in burn wound healing. Inflammation 2016;39:1395-404.



18. Belém MO, de Andrade GMM, Carlos TM, Guazelli CFS, Fattori V, Toginho Filho DO, et al. Light-emitting diodes at 940nm attenuate colitis-induced inflammatory process in mice. J Photochem Photobiol B 2016;162:367-73.


19. Goldberg DJ, Amin S, Russell BA, Phelps R, Kellett N, Reilly LA. Combined 633-nm and 830-nm led treatment of photoaging skin. J Drugs Dermatol 2006;5:748-53.

20. Oshima Y, Coutts RD, Badlani NM, Healey RM, Kubo T, Amiel D. Effect of light-emitting diode (LED) ther-apy on the development of osteoarthritis (OA) in a rabbit model. Biomed Pharmacother 2011;65:224-9.


21. Chen IC, Chen-Ying S, Chi-Hau F, Hsu-Wei F. Preventative treatment of red light-emitting diode protected osteoarthritis-like chondrocytes from oxidative stress-induced inflammation and promoted matrix gene expression. J Taiwan Inst Chem Eng 2021;127:23-31.

22. de Morais NC, Barbosa AM, Vale ML, Villaverde AB, de Lima CJ, Cogo JC, et al. Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis. Photomed Laser Surg 2010;28:227-32.


23. Jekal SJ, Kwon PS, Kim JK, Lee JH. Effect of 840 ㎚ light-emitting diode (LED) irradiation on monosodium iodoacetate-induced osteoarthritis in rats. J Korean Soc Phys Med 2014;9:151-9.

24. Alves-Simões M. Rodent models of knee osteoarthritis for pain research. Osteoarthritis Cartilage 2022;30:802-14.


25. Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep 2016;6:33038.




26. Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol 2013;6:126-35.



27. Watanabe M, Campbell TM, Reilly K, Uhthoff HK, Laneuville O, Trudel G. Bone replaces unloaded articu-lar cartilage during knee immobilization. A longitudinal study in the rat. Bone 2021;142:115694.


28. Harro J. Animals, anxiety, and anxiety disorders: how to measure anxiety in rodents and why. Behav Brain Res 2018;352:81-93.


29. Liu S, Deng Z, Chen K, Jian S, Zhou F, Yang Y, et al. Cartilage tissue engineering: from proinflammatory and anti‑inflammatory cytokines to osteoarthritis treatments (Review). Mol Med Rep 2022;25:99.



30. Fulga C. Antiinflammatory effect of laser therapy in rheumatoid arthritis. Rom J Intern Med 1998;36:273-9.

31. Jawad MM, Husein A, Azlina A, Alam MK, Hassan R, Shaari R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J Biomed Opt 2013;18:128001.


32. Ebrahimi H, Darvish F, Alaeddini M, Etemad-Moghadam S. Comparison between the effect of 810 nm and 940 nm diode laser irradiation on histopathological changes in iatrogenic oral ulcers: an animal study. J Dent (Shiraz) 2021;22:267-72.


33. Taradaj J, Halski T, Kucharzewski M, Urbanek T, Halska U, Kucio C. Effect of laser irradiation at different wavelengths (940, 808, and 658 nm) on pressure ulcer healing: results from a clinical study. Evid Based Complement Alternat Med 2013;2013:960240.




34. Baracho VDS, Chaves MEA, Huebner R, Oliveira MX, Ferreira PHDC, Lucas TC. Phototherapy (cluster multi-diode 630 nm and 940 nm) on the healing of pressure injury: a pilot study. J Vasc Nurs 2021;39:67-75.


35. Alves AC, Vieira R, Leal-Junior E, dos Santos S, Ligeiro AP, Albertini R, et al. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther 2013;15:R116.



36. Bellido M, Lugo L, Roman-Blas JA, Castañeda S, Caeiro JR, Dapia S, et al. Subchondral bone microstruc-tural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther 2010;12:R152.



37. Botter SM, van Osch GJ, Waarsing JH, Day JS, Verhaar JA, Pols HA, et al. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology 2006;43:379-88.

38. Ma L, Zhao X, Liu Y, Wu J, Yang X, Jin Q. Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone. Int J Mol Med 2021;47:04855.










