2. Wissel J, Ri S. Assessment, goal setting, and botulinum neurotoxin a therapy in the management of post-stroke spastic movement disorder: updated perspectives on best practice. Expert Rev Neurother 2022;22:27-42.


5. Mills PB, Phadke CP, Boulias C, Dukelow SP, Ismail F, McNeil SM, et al. Spasticity management teams, evaluations, and tools: a Canadian cross-sectional survey. Can J Neurol Sci 2022. doi: 10.1017/cjn.2022.326. [Epub ahead of print].

6. Wissel J, Ward AB, Erztgaard P, Bensmail D, Hecht MJ, Lejeune TM, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med 2009;41:13-25.


8. Platz T, Vuadens P, Eickhof C, Arnold P, Van Kaick S, Heise K. REPAS, a summary rating scale for resistance to passive movement: item selection, reliability and validity. Disabil Rehabil 2008;30:44-53.


9. Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005;27:2-6.


12. Wissel J, Ri S, Kivi A. Early versus late injections of Botulinumtoxin type A in post-stroke spastic movement disorder: a literature review. Toxicon 2023;229:107150.


14. Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980;30:1303-13.


19. Chen CL, Tang FT, Chen HC, Chung CY, Wong MK. Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil 2000;81:447-52.


20. Ri S, Kivi A, Urban PP, Wolf T, Wissel J. Site and size of lesion predict post-stroke spasticity: a retrospective magnetic resonance imaging study. J Rehabil Med 2020;52:jrm00065.


21. Ri S, Glaess-Leistner S, Wissel J. Early brain imaging predictors of post-stroke spasticity. J Rehabil Med 2021;53:jrm00169.


22. Gracies JM. Pathophysiology of spastic paresis. I: paresis and soft tissue changes. Muscle Nerve 2005;31:535-51.


23. Baude M, Nielsen JB, Gracies JM. The neurophysiology of deforming spastic paresis: a revised taxonomy. Ann Phys Rehabil Med 2019;62:426-30.


26. Urban PP, Wolf T, Uebele M, Marx JJ, Vogt T, Stoeter P, et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010;41:2016-20.


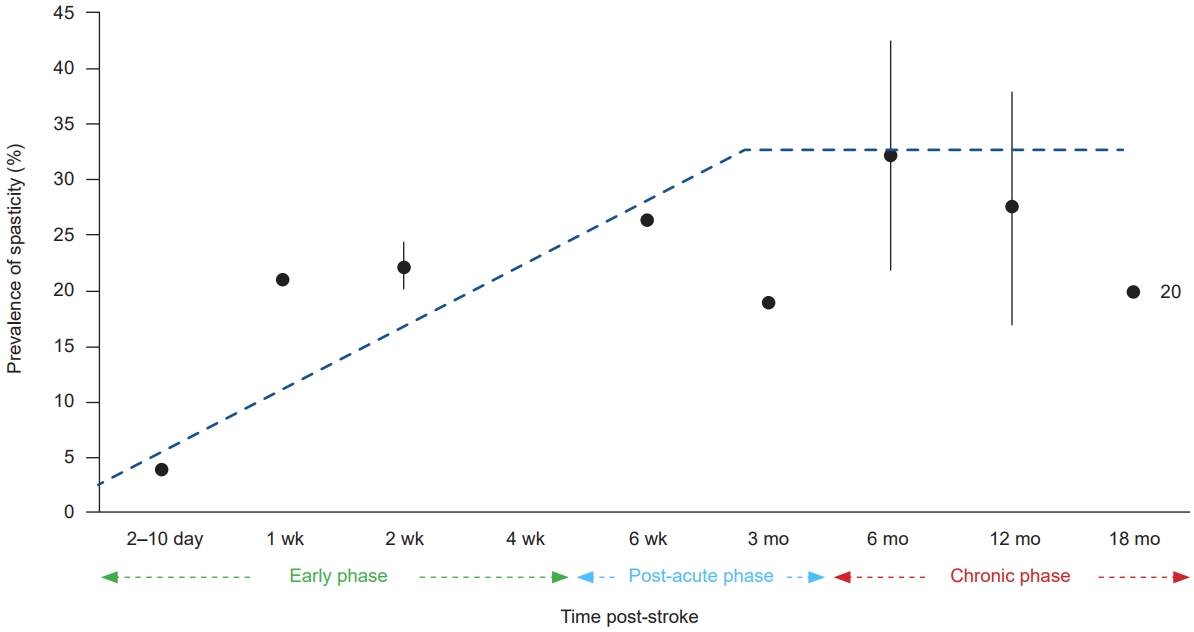
27. Lundström E, Smits A, Terént A, Borg J. Time-course and determinants of spasticity during the first six months following first-ever stroke. J Rehabil Med 2010;42:296-301.


30. Kong KH, Lee J, Chua KS. Occurrence and temporal evolution of upper limb spasticity in stroke patients admitted to a rehabilitation unit. Arch Phys Med Rehabil 2012;93:143-8.


32. Ryu JS, Lee JW, Lee SI, Chun MH. Factors predictive of spasticity and their effects on motor recovery and functional outcomes in stroke patients. Top Stroke Rehabil 2010;17:380-8.


34. Glaess-Leistner S, Ri SJ, Audebert HJ, Wissel J. Early clinical predictors of post-stroke spasticity. Top Stroke Rehabil 2021;28:508-18.


37. Glaess-Leistner S, Ri SJ, Audebert HJ, Wissel J. Early clinical predictors of post stroke spasticity. Top Stroke Rehabil 2021;28:508-18.


39. Turner-Stokes L, Baguley IJ, De Graaff S, Katrak P, Davies L, McCrory P, et al. Goal attainment scaling in the evaluation of treatment of upper limb spasticity with botulinum toxin: a secondary analysis from a double-blind placebo-controlled randomized clinical trial. J Rehabil Med 2010;42:81-9.


41. Kivi A, Ri S, Wissel J. What clinicians and patients want: the past, the presence, and the future of the botulinum toxins. Toxicon 2020;177:46-51.


42. Rosales RL, Efendy F, Teleg ES, Delos Santos MM, Rosales MC, Ostrea M, et al. Botulinum toxin as early intervention for spasticity after stroke or non-progressive brain lesion: a meta-analysis. J Neurol Sci 2016;371:6-14.


43. Varvarousis DN, Martzivanou C, Dimopoulos D, Dimakopoulos G, Vasileiadis GI, Ploumis A. The effectiveness of botulinum toxin on spasticity and gait of hemiplegic patients after stroke: a systematic review and meta-analysis. Toxicon 2021;203:74-84.


44. Fheodoroff K, Scheschonka A, Wissel J. Goal analysis in patients with limb spasticity treated with incobotulinumtoxinA in the TOWER study. Disabil Rehabil 2022;44:1367-73.


45. Karri J, Mas MF, Francisco GE, Li S. Practice patterns for spasticity management with phenol neurolysis. J Rehabil Med 2017;49:482-8.


49. Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J Biomech 2011;44:39-44.