- Search
| Ann Rehabil Med > Volume 47(5); 2023 > Article |
|
Abstract
Objective
To systematically review the effects of protein supplementation in older adults with sarcopenia.
Methods
A systematic literature search was conducted in PubMed, Cochrane Library, and Embase databases until May 2023. The inclusion criteria were as follows: (1) randomized controlled trials with a quantitative study design; (2) studies with a study group of older adults with sarcopenia; (3) studies comparing muscle mass, muscle strength, and performance of older adults with sarcopenia after protein supplementation; and (4) studies published up to May 2023.
Results
Six retrospective comparative studies, including 715 patients, met the inclusion criteria. The nutritional supplementation group exhibited significant improvement in appendicular skeletal muscle mass (standardized mean difference [SMD]=0.41; 95% confidence interval [CI], 0.24–0.58; p<0.001; I2=1%), while handgrip strength (SMD=0.37; 95% CI, -0.32–1.07; p=0.29; I2=94%) and Short Physical Performance Battery (SPPB) (SMD=0.35; 95% CI, -0.47–1.18; p=0.40; I2=94%) showed a tendency for improvement.
Age-related muscle attenuation, termed “sarcopenia,” contributes to muscle weakness and impaired physical mobility. Sarcopenia and frailty are multidimensional syndromes characterized by a decreased reserve and diminished resistance to stressors [1,2]. Criteria for classifying older adults as having sarcopenia or high frailty risk have recently been established. In 2019, the Asian Working Group for Sarcopenia (AWGS) defined sarcopenia as “age-related loss of muscle mass, plus low muscle strength, or low physical performance.” It proposed diagnostic cut-offs for each component [3]. Low muscle strength is a handgrip strength of <28 kg for male and <18 kg for female. The criteria for low physical performance are a 6-minute walk test slower than 1.0 m/s, a Short Physical Performance Battery (SPPB) score of ≤9, or a 5-time chair stand test of ≥12 seconds. Cut-offs for height-adjusted muscle mass through dual-energy X-ray absorptiometry were <7.0 kg/m2 in male and <5.4 kg/m2 in female and that through bioimpedance were <7.0 kg/m2 in male and <5.7 kg/m2 in female. Sarcopenia in older adults can be devastating, resulting in several adverse events, including increased risk for falls, impaired mobility, elderly depression, increased healthcare costs, and mortality [4,5].
Previous studies report that oral nutritional supplementation improves body composition and decreases functional disability. Among nutritional supplements, high-quality protein or amino acid supplementation is important for preventing a decrease in muscle mass. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein or casein hydrolysate in older people [6]. Whey protein is a rapidly digested dietary protein beneficial for slowing muscle loss, particularly in older adults. When protein or amino acid intake is supplemented, the amount of synthesized muscle protein exceeds the amount broken down [7]. Sufficient protein intake is crucial to maintain muscle mass and function in older adults. The PROT-AGE study group recommended at least 1.0–1.2 g protein/kg/day to maintain and increase muscle mass and function in individuals aged older than 65 years. A higher protein intake (>1.2 g/kg/day) is advised for those who regularly exercise and for community-dwelling older adults [8].
Previous systematic reviews and meta-analysis were limited to parameters related to muscle mass and investigated whether nutritional supplementation could improve muscle mass in older populations [9]. Few meta-analysis of the functional outcomes of nutritional supplementation in older adults with sarcopenia have been conducted. The meta-analysis on the effect of nutritional supplementation on elderly sarcopenia needs an update since several randomized controlled trials (RCTs) have been published after the previous meta-analysis. The management of sarcopenia should focus on increasing muscle mass and functional improvements to eventually prevent the progression of sarcopenia. This study aimed to conduct a focused meta-analysis of studies that used nutritional interventions with a protein-or amino acid-enriched formula in older adults with sarcopenia.
This meta-analysis was conducted according to the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Supplementary Material) [10].
The search was performed using PubMed, Cochrane Library, and Embase databases from inception to May 2023 using the following keywords: “older adults” AND “sarcopenia” AND “protein” OR “nutrition” AND “supplement” OR “muscle mass” OR “muscle strength” AND “short physical performance battery.”
Studies were included if they satisfied all of the following selection criteria: (1) RCTs with a quantitative study design; (2) studies with a group of older adults (age ≥65 years) with sarcopenia; (3) studies comparing muscle mass, muscle strength, and functional performance of older adults with sarcopenia after receiving adequate protein supplementation; (4) the supplement intervention used protein sources, including whey protein or leucine, vitamins, and other nutrients; and (5) studies published up to May 2023. Studies published as case reports, case series, or prospectively designed trials without comparison groups were excluded from the analysis.
Data were extracted from each included study and are presented in an evidence table (Table 1) outlining the characteristics of the respective study designs and participants (group design, age, and sex), body composition assessment methods (bioimpedance analysis or dual-energy X-ray absorptiometry), the composition of nutritional supplements, follow-up period, and main measured outcomes [11-16].
Six studies compared older individuals with sarcopenia using diagnostic criteria proposed by the AWGS as quantitative values between the group with nutritional support and the group with placebo. These studies compared muscle mass, muscle strength, and functional performance, including at least two of the following: appendicular skeletal muscle mass (ASM), handgrip strength, and SPPB score, after receiving adequate protein supplementation.
We separately computed the effect sizes for each study for the primary and secondary outcome measures. The R metapackage was used for statistical analysis and graphics (http://www.r-project.org). The heterogeneity of the studies was calculated using the I2 test to observe variations across studies and was estimated to be significant when p<0.05. The 95% confidence interval (CI) and two-tailed p-values are provided. All extracted outcome data were calculated as the standardized mean difference (SMD) compared to the control group. Fixed-effects or random-effects models were used depending on the existence of heterogeneity. A fixed-effects model was used when the statistical heterogeneity was insignificant (I2 values were ≤50%); otherwise, a random-effects model was used.
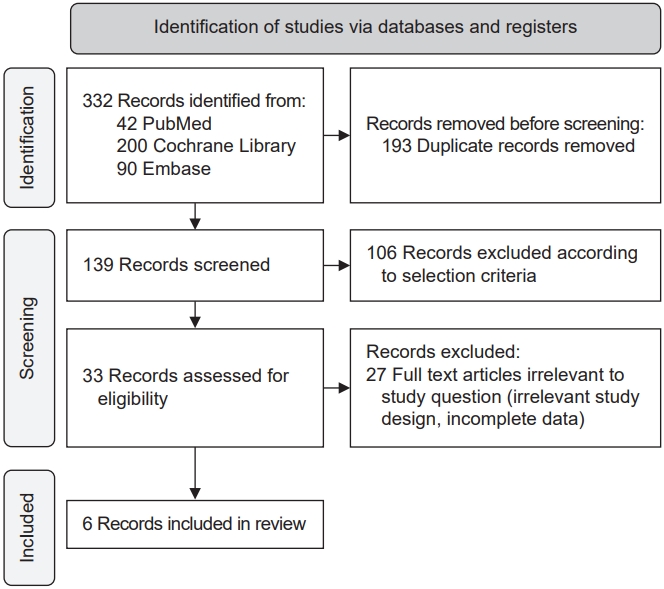
Fig. 1 shows a flowchart of the selection process. Of the 332 studies retrieved using the above-mentioned keywords, 139 were selected after excluding duplicate articles. After confirming the title and abstract, 106 articles were excluded. In addition, 27 studies could not be included in the meta-analysis because of irrelevant study design or insufficient data. Six studies were finally included in this review.
The nutritional interventions and protocols used for the exercise training are summarized in Table 1. The nutritional supplementation protocols varied widely among the included trials. The majority of the included RCTs provided extra protein supplements with amounts of whey protein ranging from 10.0 to 40.0 g/day [9]. One RCT used leucine (1 g), arginine (1.5 g), and vitamin-D 300 IU complex without whey protein. Vitamin-D levels ranged from 600 to 1,600 IU/day. One study used only whey protein as a nutritional supplement without other additives [14]. Regarding the mode of exercise, one RCT used only resistance exercise training. Four RCTs used multi-component exercise regimens. One RCT did not include an exercise program before supplement intake.
In this meta-analysis, a group of patients who received whey protein or amino acid or vitamin-D supplementation was defined as the experimental group, and a group of patients who received a placebo was defined as the control group.
In the ASM analysis, of 536 patients, 263 in the experimental group received the protein supplementation, while 273 in the control group did not. There was a significant difference between the experimental and control groups with regard to ASM (SMD=0.41; 95% CI, 0.24–0.58; p<0.001; I2=1%; Fig. 2).
In the analysis of handgrip strength, of 715 patients, 351 in the experimental group received the protein supplementation, while 364 in the control group did not. There was no statistically significant difference between the experimental and control groups in handgrip strength (SMD=0.37; 95% CI, -0.32–1.07; p=0.29; I2=94%; Fig. 3A).
In the analysis of the SPPB scores, among 667 patients, 327 in the experimental group received the protein supplementation, while 340 in the control group did not. There was no statistically significant difference between the experimental and control groups in SPPB scores (SMD=0.35; 95% CI, -0.47–1.18; p=0.40; I2=94%; Fig. 3B).
In terms of methodological quality, all participants were randomized using established allocation sequences. Of the 42 domains among all studies, 31 domains (73.8%) were of low risk. Therefore, the overall risk of bias was determined to be low, and the studies included in this meta-analysis were assessed as of high quality (Fig. 4).
The funnel plot for the ASM was symmetrical. In contrast, the graphic funnel plots for handgrip strength and SPPB scores were asymmetrical (Fig. 5). In addition, Egger’s linear regression test indicated an insignificant publication bias for ASM (p=0.12), handgrip strength (p=0.82), as well as SPPB (p=0.69).
In this meta-analysis, protein supplementation significantly increased ASM in older adults with sarcopenia and tended to improve functional outcomes such as handgrip strength and SPPB scores. Sarcopenia is the progressive loss of muscle mass, strength, and function related to aging [17]. In older adults, chronic inflammation, motor neuron atrophy, reduced protein intake, and immobility can contribute to the progression of sarcopenia [18]. Furthermore, managing sarcopenia in older adults is crucial as it could lead to fatal adverse events, including increased risk for falls, impaired mobility, increased healthcare costs, and mortality [4].
In our study, the ASM increased significantly after protein supplementation (SMD=0.41). The groups in the six studies were homogeneous, and meaningful conclusions could be drawn (I2=1%). These findings are consistent with those of a previous study showing that whey protein, leucine, or vitamin-D-enriched supplementation in patients with sarcopenia increased appendicular muscle mass [19]. In contrast, one RCT reported that protein supplementation does not increase ASM in older patients with sarcopenia but improves physical performance [20]. However, this study had some limitations as it did not implement the exercise therapy with nutritional support, in addition to a smaller sample size (n=65) compared to that in our study.
Previous studies reporting the effect of nutritional supplements on sarcopenia mainly concluded with primary outcome measures of sarcopenia indices, including lean body mass, appendicular lean mass, or skeletal mass index [21,22]. However, there were few studies with a large sample size whose outcome measures of functional performance were SPPB, gait speed, or chair-stand test [3].
Handgrip strength and performance scored with SPPB tended to improve, although this was not statistically significant (SMD=0.37; SMD=0.35). Another meta-analysis showed that changes in appendicular lean mass were significantly associated with leg strength and walking capability [23]. The heterogeneity of the participant groups included in the muscle strength and performance evaluation may have increased the variance in this study. As this study did not confirm a change in muscle mass affecting muscle strength and functional mobility, further studies are needed. Handgrip strength is a diagnostic tool for sarcopenia in the geriatric population and is known to reflect sarcopenic conditions better than other measurement tools, such as the chair-stand test [24]. Low handgrip strength could be a main predictor of mortality and adverse events in older individuals with sarcopenia [25]. In fixed-effect model, we observed a tendency for handgrip strength to improve after protein supplementation (SMD=0.29; p<0.001). Protein supplementation could increase muscle mass and improve physical function. This could eventually lead to reduced mortality and risk for falls and a better quality of life in older adults with sarcopenia [26]. Considering the heterogeneity, further studies with homogenous groups are needed.
The SPPB is suggested as a good alternative for gait speed to assess physical performance in sarcopenia. The SPPB consists of three tests for lower limb function, balance, strength, and mobility and is a comprehensive evaluation of functional mobility. The SPPB score predicts long-term mortality [27] and evaluates the ability to perform resistance and aerobic exercises to prevent sarcopenia. This study showed that protein supplementation would increase SPPB scores in fixed-effect model (SMD=0.32; p<0.001). Improvements in SPPB scores are expected to prevent frailty, hospitalization, and mortality in older adults [28].
In our analysis, studies involving the use of protein supplements and whey proteins were the primary focus. Selecting high-quality proteins as nutritional supplements could effectively increase muscle mass and functional performance. Such supplements are also easy for older adults to digest, considering the physiological and metabolic changes that occur with aging [29]. Whey protein affects muscle strengthening differently in older individuals than that in young individuals. Whey protein, a rapidly digested protein, is utilized more when consumed with other carbohydrates, especially in older adults. The addition of amino acids, or specifically, leucine, is also recommended [30].
This study showed that protein supplementation increased ASM, regardless of the type, duration, and intensity of exercise. Protein supplementation and muscle-strengthening exercises contribute to increased muscle strength and walking capability [23]. Aerobic and resistance exercises reduced the time interval between muscle protein breakdown and synthesis. Therefore, a protein nutritional supplement and a concomitant aerobic or resistance exercise program can be recommended to reduce the rate of muscle loss. In addition, early exercise and nutritional intervention could be helpful in an earlier restoration of lower extremity muscle mass for sarcopenia in older adults. Appropriate early resistance training with nutritional support and subsequent structuralized home-based exercise should be administered [31].
This study has several limitations. The heterogeneity of the intervention regimen made it difficult to conclude the effectiveness of each protocol due to the variation among protein supplement regimens (protein source, supplied amounts, and timing of ingestion) and exercise regimens (type of training, training duration, and training volume). Among the six studies we selected, the functional mobility assessment protocols were inconsistent. This resulted in the extraction of only limited valid data. However, we attempted to select the main assessment tools for sarcopenia, such as handgrip strength and SPPB. Additionally, the follow-up was limited to a short-term period (12 weeks to 1 year). Well-designed RCTs with longer follow-up periods could help establish the long-term effect of protein supplementation in older patients with sarcopenia.
In conclusion, protein supplementation significantly increases appendicular muscle mass in older patients with sarcopenia and could lead to improvements in functional outcomes, such as handgrip strength and SPPB scores. Therefore, sufficient protein supplementation may be crucial for managing sarcopenia in older individuals.
AUTHOR CONTRIBUTION
Conceptualization: Kwon HE, Koh SE. Methodology: Kwon HE, Ko N. Formal analysis: Kwon HE, Yuk D, Choi SW. Project administration: Kwon HE, Ko N. Visualization: Kwon HE, Yuk D, Choi SW. Writing – original draft: Kwon HE, Ko N. Writing – review and editing: Kwon HE, Ko N, Yuk D, Koh SE. Approval of final manuscript: all authors.
Fig. 2.
Forest plot showing the results of appendicular muscle mass in sarcopenia after protein supplementation. SD, standard deviation; SMD, standardized mean difference; 95% CI, 95% confidence interval.
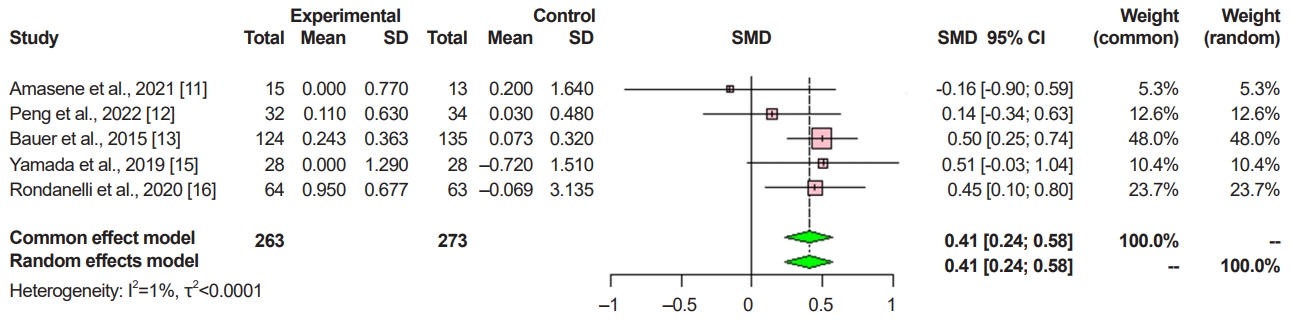
Fig. 3.
Forest plot showing the results of (A) handgrip strength and (B) Short Physical Performance Battery (SPPB) scores after protein supplementation. SD, standard deviation; SMD, standardized mean difference; 95% CI, 95% confidence interval.

Fig. 4.
Summary of the quality assessment of the randomized controlled trials included in the meta-analysis.

Fig. 5.
Funnel plot of the included studies of (A) appendicular skeletal muscle mass (ASM), (B) handgrip strength, and (C) Short Physical Performance Battery (SPPB) scores.
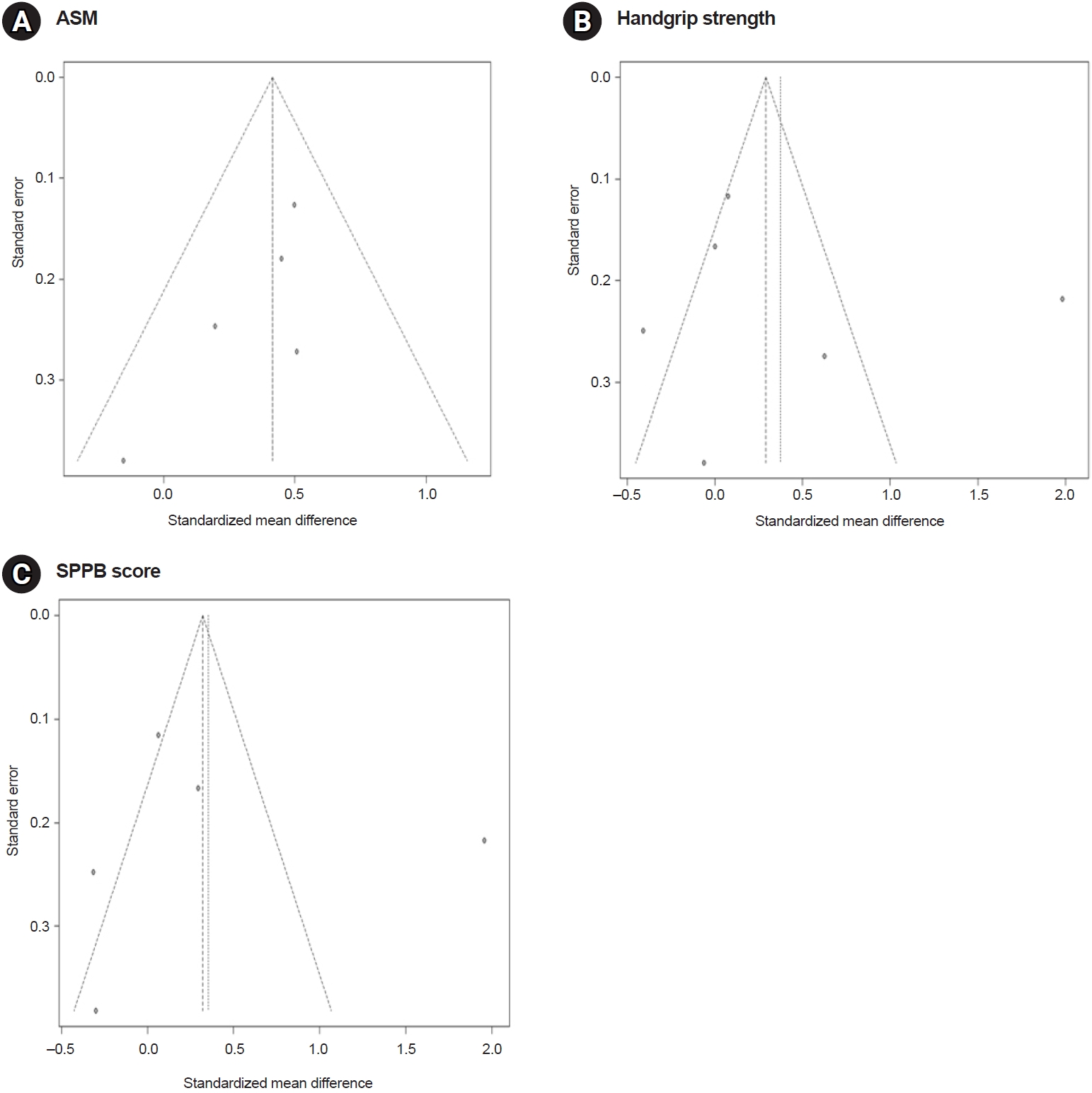
Table 1.
A summary of selected studies’ characteristics
| Study | Study design | Group | Age (yr) | Sex (female/male) | Body composition assessment method | Intervention (nutritional supplementation/exercise) | Follow-up period | Measured outcomes |
|---|---|---|---|---|---|---|---|---|
| Amasene et al., 2021 [11] | Randomized controlled trial | Intervention (N=21) | 82.9±5.67 | 12/9 | DXA | 20 g of whey protein isolate enriched with 3 g of leucine | 12 wk | ASM, handgrip strength, SPPB (balance, gait speed, chair stand), weight, BMI, calf circumference, body fat, lean mass, fat-free mass and bone mass, myostatin, follistatin, irisin |
| Control (N=20) | 81.2±6.14 | 10/10 | Supervised resistance training (3 times a week) | |||||
| Peng et al., 2022 [12] | Randomized controlled trial | Intervention (N=12) | 67.1±6.7 | 8/4 | DXA | Leucine 1 g, arginine 1.5 g, Vitamin D3 7.5 μg, chondroitin 400 mg, glucosamine 700 mg, and calcium 300 mg twice a day | 12 wk | ASM, handgrip strength, 6-minute walk test, chair stand test, SPPB, MMSE |
| Control (N=13) | 68.9±6.2 | 11/2 | 45 minutes at the gym per week and two sessions of 30-minute exercise at home | Serum levels of the total protein, albumin, hepatic and renal function tests, blood lipids, glucose, insulin, HbA1c, electrolytes, and hS-CRP, 25-hydroxyvitamin D | ||||
| Bauer et al., 2015 [13] | Randomized controlled trial | Intervention (N=184) | 77.3±6.7 | 120/64 | DXA | 20 g whey protein, 3 g total leucine, 9 g carbohydrates, 3 g fat, 800 IU vitamin D, and a mixture of vitamins, minerals, and fibers twice daily | 7, 13 wk | ASM, hand grip strength, SPPB, chair-stand test, gait speed, balance test, serum 25-hydroxyvitamin D, serum IGF-1 |
| Control (N=196) | 78.1±7.0 | 129/67 | ||||||
| Björkman et al., 2020 [14] | Randomized controlled trial | Intervention (N=73) | 83.6±4.7 | 51/21 | BIA | 20 g whey proteins twice a day | 12 mo | Hand grip strength, SPPB, continuous summary physical performance scores, SMI, MNA, dietary record, MMSE |
| Control (N=73) | 83.7±5.1 | 129/67 | Given instructions on home-based exercise | |||||
| Yamada et al., 2019 [15] | Randomized controlled trial | Intervention (N=28) | 84.9±5.6 | 20/8 | BIA | 10.0 g of whey proteins and 800 IU vitamin D | 12 wk | ASM, handgrip strength, knee extension torque, phase angle, echo intensity for rectus femoris, gait speed, one-leg standing time, chair-stand time |
| Control (N=28) | 83.9±5.7 | 15/13 | 30 minutes of body weight resistance exercise with slow movement speeds twice a week | |||||
| Rondanelli et al., 2020 [16] | Randomized controlled trial | Intervention (N=28) | 81±7.0 | 38/26 | BIA | 20 g of whey proteins, 2.8 g of leucine, 9 g of carbohydrates, 3 g of fat, 800 IU of vitamin D, and a mixture of vitamins, minerals (calcium 500 mg), and fibers | 4–8 wk | ASM, SMI, change in 4-m gait speed per month, handgrip strength, SPPB, chair-stand test, timed up and go test, body weight, MNA, CRP, serum 25-hydroxyvitamin D, cholesterol, albumin, creatinine |
| Control (N=28) | 82±5.0 | 46/17 | 20–30-minute session; muscle-strengthening exercises, balance, gait exercises |
DXA, dual-energy X-ray absorptiometry; ASM, appendicular skeletal muscle mass; SPPB, Short Physical Performance Battery; BMI, body mass index; MMSE, Mini Mental State Examination; HbA1c, whole-blood glycated hemoglobin; hS-CRP, high-sensitivity C-reactive protein; IGF-1, insulin-like growth factor 1; BIA, bioimpedance analysis; SMI, skeletal muscle mass index; MNA, Mini Nutritional Assessment.
REFERENCES
1. Lee SY, Lee HJ, Lim JY. Effects of leucine-rich protein supplements in older adults with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Arch Gerontol Geriatr 2022;102:104758.


2. Saad F. The relationship between testosterone deficiency and frailty in elderly men. Horm Mol Biol Clin Investig 2010;4:529-38.


3. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300-7.e2.


4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636-46. Erratum in: Lancet 2019;393:2590.


5. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017;46:738-46.


6. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 2011;93:997-1005.


7. Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 1997;273(1 Pt 1): E122-9.


8. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14:542-59.


9. Wright J, Baldwin C. Oral nutritional support with or without exercise in the management of malnutrition in nutritionally vulnerable older people: a systematic review and meta-analysis. Clin Nutr 2018;37(6 Pt A): 1879-91.


10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. W64.


11. Amasene M, Cadenas-Sanchez C, Echeverria I, Sanz B, Alonso C, Tobalina I, et al. Effects of resistance training intervention along with leucine-enriched whey protein supplementation on sarcopenia and frailty in post-hospitalized older adults: preliminary findings of a randomized controlled trial. J Clin Med 2021;11:97.



12. Peng LN, Yu PC, Hsu CC, Tseng SH, Lee WJ, Lin MH, et al. Sarcojoint®, the branched-chain amino acid-based supplement, plus resistance exercise improved muscle mass in adults aged 50 years and older: a double-blinded randomized controlled trial. Exp Gerontol 2022;157:111644.


13. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015;16:740-7.


14. Björkman MP, Suominen MH, Kautiainen H, Jyväkorpi SK, Finne-Soveri HU, Strandberg TE, et al. Effect of protein supplementation on physical performance in older people with sarcopenia-a randomized controlled trial. J Am Med Dir Assoc 2020;21:226-32.e1.


15. Yamada M, Kimura Y, Ishiyama D, Nishio N, Otobe Y, Tanaka T, et al. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr Gerontol Int 2019;19:429-37.



16. Rondanelli M, Cereda E, Klersy C, Faliva MA, Peroni G, Nichetti M, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle 2020;11:1535-47.




17. Rizzoli R, Reginster JY, Arnal JF, Bautmans I, Beaudart C, Bischoff-Ferrari H, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013;93:101-20.




18. Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas 2012;71:109-14.


19. Chang MC, Choo YJ. Effects of whey protein, leucine, and vitamin d supplementation in patients with sarcopenia: a systematic review and meta-analysis. Nutrients 2023;15:521.



20. Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720-6.


21. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med 2016;31:643-50.




22. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69:567-75.



23. Liao CD, Chen HC, Huang SW, Liou TH. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: a systematic review and meta-regression analysis of randomized trials. Nutrients 2019;11:1713.



24. Verstraeten LMG, de Haan NJ, Verbeet E, van Wijngaarden JP, Meskers CGM, Maier AB. Handgrip strength rather than chair stand test should be used to diagnose sarcopenia in geriatric rehabilitation inpatients: REStORing health of acutely unwell adulTs (RESORT). Age Ageing 2022;51:afac242.




25. Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ 2010;182:429-35.



26. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2019;10:485-500.




27. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85-94.


28. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 2017;12:e0169548.



29. Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, et al. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol 2003;549(Pt 2): 635-44.




- TOOLS