- Search
| Ann Rehabil Med > Volume 47(4); 2023 > Article |
|
Abstract
Objective
Methods
Results
AUTHOR CONTRIBUTION
Conceptualization: Cho TH, Ma MK, Moon HI. Methodology: Ma MK, Cho TH, Moon HI. Formal analysis: Ma MK, Cho TH, Lee JW, Moon HI. Project administration: Moon HI, Ma MK. Visualization: Ma MK, Cho TH, Lee JW. Writing – original draft: Ma MK, Cho TH. Writing – review and editing: Moon HI, Ma MK. Approval of final manuscript: all authors.
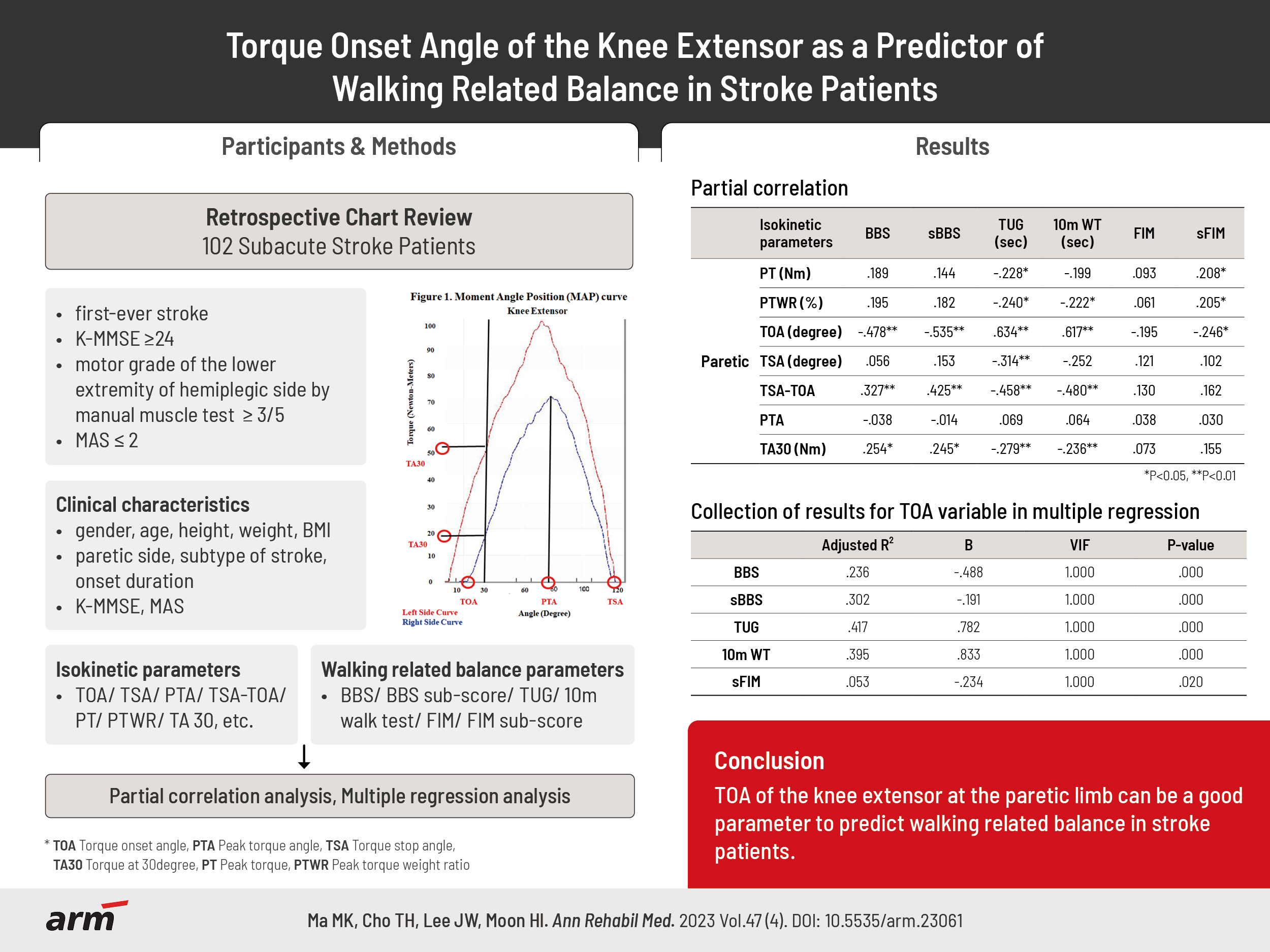
Fig. 1.
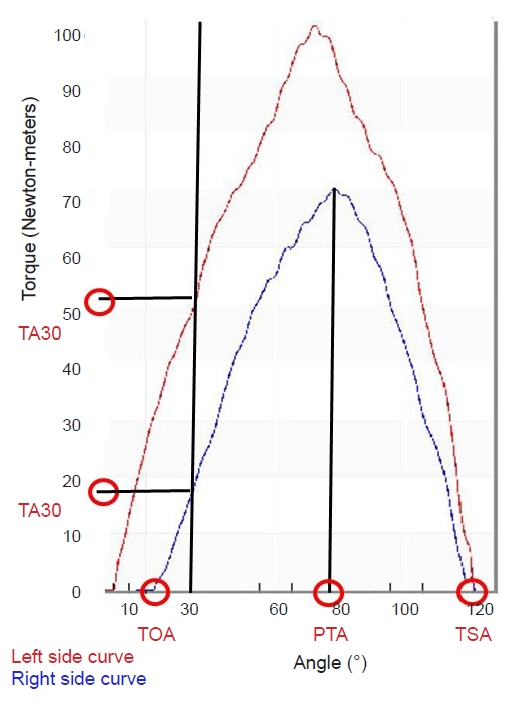
Table 1.
Values are number only or mean±standard deviation (range).
K-MMSE, Korean version of the Mini-Mental State Examination; MAS, Modified Ashworth Scale (MAS grades of 1 was considered as 1, while a score of 1+ was considered as 2 and so on up to a score of 4, which was considered as 5); PT_N, non-paretic peak torque; PTWR_N, non-peak torque weight ratio; PT_P, paretic peak torque; PTWR_P, paretic peak torque weight ratio; PT deficit, non-paretic peak torque-paretic peak torque.
Table 2.
| Isokinetic parameters | BBS | sBBS | TUG (s) | 10MWT (s) | FIM | sFIM |
|---|---|---|---|---|---|---|
| Non-paretic | ||||||
| PT (Nm) | 0.194 | 0.068 | -0.208* | -0.181 | 0.207* | 0.203* |
| PTWR (%) | 0.221* | 0.108 | -0.252* | -0.204* | 0.155 | 0.253* |
| TW (J) | 0.219* | 0.108 | -0.219* | -0.199* | 0.189 | 0.165 |
| HPT (Nm) | 0.071 | -0.033 | -0.110 | -0.081 | 0.232* | 0.186 |
| H/Q ratio | -0.178 | -0.184 | 0.165 | 0.178 | 0.055 | -0.006 |
| TOA (°) | 0.101 | 0.042 | -0.020 | 0.040 | 0.027 | 0.053 |
| TSA (°) | -0.096 | -0.076 | 0.053 | 0.029 | -0.070 | -0.104 |
| TSA-TOA | 0.038 | -0.012 | -0.025 | -0.097 | -0.048 | 0.010 |
| PTA | -0.134 | -0.148 | 0.137 | 0.140 | -0.073 | -0.146 |
| TA30 (Nm) | 0.160 | 0.104 | -0.115 | -0.131 | 0.077 | 0.072 |
| Paretic | ||||||
| PT (Nm) | 0.242* | 0.197* | -0.306** | -0.280** | 0.191 | 0.213* |
| PTWR (%) | 0.281** | 0.262** | -0.358** | -0.317** | 0.129 | 0.227* |
| TW (J) | 0.297** | 0.249* | -0.360** | -0.337** | 0.174 | 0.216* |
| HPT (Nm) | 0.226* | 0.228* | -0.302** | -0.324** | 0.215* | 0.225* |
| H/Q ratio | 0.052 | 0.118 | -0.031 | -0.107 | 0.040 | 0.049 |
| TOA (°) | -0.493** | -0.556** | 0.646** | 0.629** | -0.195* | -0.229* |
| TSA (°) | 0.019 | 0.140 | -0.279** | -0.246* | 0.050 | 0.056 |
| TSA-TOA | 0.279** | 0.402** | -0.439** | -0.470** | 0.051 | 0.119 |
| PTA | -0.024 | -0.006 | 0.015 | 0.046 | 0.064 | 0.030 |
| TA30 (Nm) | 0.308** | 0.292** | -0.332** | -0.302** | 0.119 | 0.158 |
| Asymmetry | ||||||
| PT deficit | -0.077 | -0.212* | 0.160 | 0.160 | 0.030 | -0.012 |
| PT ratio | 0.205* | 0.300** | -0.353** | -0.328** | 0.096 | 0.136 |
| TA30 ratio | 0.314** | 0.339** | -0.420** | -0.351** | 0.153 | 0.207* |
BBS, Berg Balance Scale; sBBS, BBS sub-score; TUG, Timed Up and Go; 10MWT, 10-m Walk Test; FIM, Functional Independence Measure; sFIM, FIM sub-score; PT, peak torque; PTWR, peak torque weight ratio; TW, total work; HPT, hamstring peak torque; H/Q ratio, hamstring/quadriceps ratio; TOA, torque onset angle; TSA, torque stop angle; PTA, peak torque angle; TA30, torque at 30°; PT deficit, non-paretic peak torque-paretic peak torque; PT ratio, (non-paretic peak torque/paretic peak torque)×100; TA30 ratio, (non-paretic torque at 30/paretic torque at 30)×100.
Table 3.
| Isokinetic parameters | BBS | sBBS | TUG (s) | 10MWT (s) | FIM | sFIM |
|---|---|---|---|---|---|---|
| Paretic | ||||||
| PT (Nm) | 0.189 | 0.144 | -0.228* | -0.199 | 0.093 | 0.208* |
| PTWR (%) | 0.195 | 0.182 | -0.240* | -0.222* | 0.061 | 0.205* |
| TOA (°) | -0.478** | -0.535** | 0.634** | 0.617** | -0.195 | -0.246* |
| TSA (°) | 0.056 | 0.153 | -0.314** | -0.252 | 0.121 | 0.102 |
| TSA-TOA | 0.327** | 0.425** | -0.458** | -0.480** | 0.130 | 0.162 |
| PTA | -0.038 | -0.014 | 0.069 | 0.064 | 0.038 | 0.030 |
| TA30 (Nm) | 0.254* | 0.245* | -0.279** | -0.236** | 0.073 | 0.155 |
| Asymmetry | ||||||
| PT ratio | 0.185 | 0.280** | -0.326** | -0.305** | 0.090 | 0.136 |
| TA30 ratio | 0.292** | 0.316** | -0.401** | -0.337** | 0.128 | 0.220* |
BBS, Berg Balance Scale; sBBS, subscale of BBS; TUG, Timed Up and Go; 10MWT, 10-m Walk Test; FIM, Functional Independence Measure; sFIM, sub-scale of FIM; PT, peak torque; PTWR, peak torque weight ratio; TOA, torque onset angle; TSA, torque stop angle; PTA, peak torque angle; TA30, torque at 30°; PT ratio, (non-paretic peak torque/paretic peak torque)×100; TA30 ratio, (non-paretic torque at 30/paretic torque at 30)×100.
Table 4.
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,575 View
- 47 Download
- Related articles in ARM
-
Isokinetic Test of the Knee Extensors and Flexors in the Healthy Twenties1986 December;10(2)