- Search
| Ann Rehabil Med > Volume 46(5); 2022 > Article |
|
Abstract
Objective
To characterize the repetitive transcranial magnetic stimulation (rTMS) induced changes in angiogenic mechanisms across different brain regions.
Methods
Seventy-nine adult male Sprague-Dawley rats were subjected to a middle cerebral artery occlusion (day 0) and then treated with 1-Hz, 20-Hz, or sham stimulation of their lesioned hemispheres for 2 weeks. The stimulation intensity was set to 100% of the motor threshold. The neurological function was assessed on days 3, 10, and 17. The infarct volume and angiogenesis were measured by histology, immunohistochemistry, Western blot, and real-time polymerase chain reaction (PCR) assays. Brain tissue was harvested from the ischemic core (IC), ischemic border zone (BZ), and contralateral homologous cortex (CH).
Results
Optical density of angiopoietin1 and synaptophysin in the IC was significantly greater in the low-frequency group than in the sham group (p=0.03 and p=0.03, respectively). The 1-Hz rTMS significantly increased the level of Akt phosphorylation in the BZ (p<0.05 vs. 20 Hz). Endothelial nitric oxide synthase phosphorylation was increased in the IC (p<0.05 vs. 20 Hz), BZ (p<0.05 vs. 20 Hz), and CH (p<0.05 vs. 20 Hz and p<0.05 vs. sham). Real-time PCR demonstrated that low-frequency stimulation significantly increased the transcriptional activity of the TIE2 gene in the IC (p<0.05).
The therapeutic potential of repetitive transcranial magnetic stimulation (rTMS) has been studied in a range of conditions, such as central pain [1,2], depression [3], migraine [4], and stroke [5–7]. The suggested mechanisms underlying the effects of rTMS include long-term potentiation and a long-term depression-like effect [8], a transient shift in the ionic balance [9], metabolic changes [10], changes in the release of neurotransmitters [11], and an enhancement of neuroprotection [12]. Repetitive TMS has been reported to produce a coupled neurovascular response in cat brains [13], implying that it may affect the vascular system. Recent studies have suggested that modulation of gene expression may be the mechanism underlying the therapeutic effect of rTMS [14,15].
Angiogenesis is as an essential mechanism underlying functional recovery after stroke [16–18]. Although angiogenic mechanisms lie dormant in adult mammalian brains, ischemic insult evokes capillary sprouting and new vessel formation in the ischemic border zones [19,20]. A significant correlation between the degree of angiogenesis and survival time in human stroke victims has been reported [21]. The increased angiogenesis induced by pharmacologic therapies appears to be related to improvements in functional outcomes [22].
Recent studies have reported that rTMS increases angiogenesis [15,23]. However, angiogenesis involves the expression of many genes, which have not all been extensively studied in previous studies. Therefore, this study sought to evaluate the effect of rTMS on angiogenic mechanisms in the early subacute phase after stroke.
All procedures described below were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (Approval no. IACUC 08-0250).
Seventy-nine adult male, 7-week-old, Sprague-Dawley rats (180–220 g) were used. The rats were allowed 1 week to adapt to the new environment in the housing facility (2–3 rats/cage, 50% relative humidity, 12-hour light/dark cycle).
Inhalation anesthesia was maintained throughout the course of the experiment. Initially, anesthesia was induced and maintained with 5% and 2%–3% isoflurane dissolved in 40%/60% and 25%/75% oxygen/nitrogen administered via a chamber and nose cone, respectively. The depth of anesthesia was adjusted to the level that abolished abdominal contractions to a tail pinch. The body temperature was monitored and maintained using a homeothermic blanket with a rectal probe (Harvard Apparatus, Holliston, MA, USA). A permanent right middle cerebral artery occlusion (MCAO) was produced using a protocol originally described by Longa et al. [24] with minor modifications.
The rats were subjected to MCAO (day 0). On POD 3, they were classified into mild (Garcia score ≥15) or severe (Garcia score <15) disability groups based on a neurological functional test. The animals in each disability group were randomly assigned to the following three treatment groups: low-frequency (1 Hz), high-frequency (20 Hz), or sham stimulation. The animals were then treated with the assigned protocols on their lesioned hemispheres for 2 weeks. Stimulation was provided using a figure-of-eight coil with the intensity set to 100% of the motor threshold. The behavioral performance was tested on POD 3, 10, and 17. Twenty-two rats in the severe disability group were sacrificed on POD 17 for subsequent analyses (Fig. 1).
The infarct volume, angiogenesis, angiogenic factor expression, and angiogenesis-related gene transcription were measured by histology, immunohistochemistry, Western blot, and real-time polymerase chain reaction (PCR), respectively. To label the proliferating cells, 10 rats—sham (n=3), 20 Hz (n=3), and 1 Hz (n=4)—in the severe disability group were injected intraperitoneally with 100 mg/kg of 5-Bromo-2’-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, Mo, USA) mixed with sterile phosphate buffer saline (PBS) on POD 3–15.
The motor threshold was determined with the center of the coil positioned 0.5 cm lateral to the bregma and with the surface placed flat onto the calvarium. Stimulation was applied using a repetitive stimulator (Magstim Rapid; Magstim Company Ltd., Wales, UK) that delivers a biphasic stimulus via a 70-mm figure-of-eight coil (model 9925-00; Magstim Company Ltd.). The maximal magnetic field strength for the coil was 2.2 T. The magnetic coil was firmly mounted on a built-in holder. The methodology was as described in a previously published study [25].
The animals were subjected to a single session of 20-minute unilateral rTMS to the lesioned hemisphere. The stimulation intensity was set to 100% of the motor threshold. The center of the coil was placed 1 cm lateral to the bregma and angulated at 45º to the ground to minimize the rTMS effect on the contralateral cortex. Subsequently, the animals were subjected to unilateral rTMS using the protocol of 20 minutes per session for the right cerebral hemisphere for 2 consecutive weeks (on POD 3–7 and 10–14).
On POD 3, 10, and 17, all the animals were evaluated using the motor behavior score developed by Garcia et al. [26], modified foot fault test, and Rotarod performance test.
Garcia’s motor behavior score is composed of six items. The scores range from 3 to 18, with a higher score reflecting better motor performance.
In the modified foot fault test, a rat was placed on the grid and encouraged to traverse the grid surface for 1 minute. The occasional errors in limb placement during movement were counted. These mistakes were considered foot faults and the number of errors per meter was assessed.
The Rotarod performance test was performed using a 9-cm diameter rod. After 2-minute adaptation at 2 rpm, the rotational speed was increased gradually by 1 rpm every minute. The rotational speed was recorded when a rat fell off the rod or just hung on without walking. This test was carried out in triplicate, and the fastest rotational speed was analyzed.
On POD 17, 10 rats—sham (n=3), 20 Hz (n=3), and 1 Hz (n=4)—in the severe disability group were deeply anesthetized and transcardially perfused with 50 mL of cold saline and another 50 mL of 4% paraformaldehyde in 0.1 mol/L PBS. The brains were harvested, fixed in 10% neutral buffered formalin, dehydrated in a graded series of ethanol, and embedded in paraffin.
For immunohistochemical analyses, a standard paraffin block was obtained from the center of the lesion. A series of 6 μm-thick sections was cut from the block. Two representative sections were used for immunostaining. The antibodies against BrdU, a proliferating cell marker (1:100; Boehringer Mannheim, Indianapolis, IN, USA), von Willebrand factor (vWF; 1:400; DAKO, Carpinteria, CA, USA), VEGF (1:100; Abcam, Cambridge, MA, USA), and Ang1 (1:2000; Abcam) were used.
Four coronal sections of the tissue were processed and stained with hematoxylin and eosin to estimate the infarct volume. The stained sections were digitized using a 20× objective (Leica DFC290; Leica Microsystems, Heerbrugg, Germany) on the Leica Application Suite (version 3.3.0) computer imaging analysis system. The indirect lesion volume was calculated and averaged by subtracting the intact brain area of the ipsilesional hemisphere from the total area of the contralesional hemisphere. The infarct volume was presented as a volume percentage of the lesion compared with the contralateral hemisphere.
The areas were calculated using ImageJ (National Institute of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij).
The number of BrdU-positive cells in two sections and 15 views in each section—five views each in the ischemic core (IC), border zone (BZ), and contralateral homologous cortex (CH)—was counted using Leica QWin standard version 3.5.1.
Five views each in the IC, BZ, CH, and corpus callosum were captured in gray scale. The optical densities (ODs) of angiopoietin 1 and synaptophysin, as markers of angiogenesis and synaptogenesis, respectively, were measured in five rectangles using MCID Analysis Evaluation version 7.0 (Imaging Research Inc., Catharines, Canada) for each region, averaged, and divided by the average OD of the corpus callosum.
The remaining 12 rats—sham (n=4), high-frequency (n=4), and low-frequency (n=4)—in the severe disability group were deeply anesthetized and decapitated 5 minutes after completion of the behavioral tests. The brain tissues were then harvested from the left cortex, left striatum, and homologous right cortex. The extracted tissues were placed in 10 volumes of cold single detergent buffer (50 MM Tris, 120 mM NaCl, pH 7.4) with protease inhibitors. Western blotting was performed using 10 μg per line of homogenized tissue for phospho-Akt, phospho-eNOS, VEGF, and GAPDH. The molecular weight was controlled using a pre-stained protein ladder (color pre-stained protein marker; ELPIS-BIOTECH, Daejeon, Korea). The protein samples were separated by 8% (for VEGF) and 12% (for Akt, eNOS, and GAPDH) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) and electrotransferred onto PVDF membranes. The blots were blocked in Tris-buffered saline (in mM, with 50 Trizma base, 150 NaCl, pH 7.4, 0.1% Tween-20, and 5% dry skimmed milk), and then incubated for 2 hours at 4°C with the primary antibodies against phospho-Akt (Ser473, Cell signaling, mouse, 1:1000), phosphor-eNOS (Ser1177, Cell signaling, rabbit, 1:1000), GAPDH (Abcam, mouse, 1:1000), and VEGF (Abcam, mouse, 1:1000). GAPDH was used as the loading control and was processed on the previously used membranes. Following the rinses, the blots were incubated for 1 hour at room temperature with an alkaline-phosphatase-conjugated secondary antibody anti-rabbit or anti-mouse IgG (1:2000, Cell signaling). The specific proteins were visualized using ECL Plus Western Blotting Detection Reagents (GE Healthcare UK Limited, Buckinghamshire, UK). The film autoradiograms were exposed for 5 to 30 minutes. The optical density was quantified from the films using ImageJ.
Total RNA was extracted from the brain tissues using the RNeasy Lipid Tissue Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. Reverse transcription was performed with 1 μg of the total RNA in a total volume of 20 μL using a Reverse Transcription Kit (Invitrogen, Waltham, MA, USA). Real-time PCR amplification was performed using an ABI Prism 7000 Sequence Detection system (Perkin-Elmer Applied Biosystems, Lincoln, CA, USA). TaqMan Universal PCR Master Mix (Applied Biosystems) and the specific primers and FAM-labeled probe sets (ProbeLibrary; Roche Applied Science, Penzberg, Germany) were used to quantify gene expression. The target genes of the specific primers were VEGF (VEGFA), Ang 1 (ANGPT1), and Tie-2 (TEK). Each sample was tested in triplicate, and the relative gene expression data were analyzed using the 2−ΔΔCT method. Gene expression levels were normalized to the internal GAPDH primer/probe pair levels (VIC/MGB probe, primer limited). Values are presented as the relative expression levels.
SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. A repeated-measure analysis of the covariance (RM-ANCOVA) was used to compare the functional outcome across the treatment groups. Comparisons were performed across treatment groups according to time for significant interactions or significant treatment effects. Kruskal-Wallis test was used to compare lesion volume and number of BrdU-positive cells. The results of Western blot were referenced to the OD of GAPDH as the control and analyzed using an ANOVA test. If an overall treatment group effect was detected at p<0.05, a Mann-Whitney U test was conducted for a pairwise comparison. All data are reported as the mean±standard error of the mean.
The results of Garcia’s motor behavior score, modified foot fault test, and the Rotarod test scores are presented in Table 1. Analysis using ANOVA showed no significant differences between the treatment groups (Fig. 2). Subgroup analysis by level of disability, mild (Garcia score ≥15) or severe (Garcia score <15), revealed continuous improvement on the modified foot fault test in the severe disability group, only with low-frequency stimulation (Supplementary Fig. S1).
There was no significant difference in the infarct volume across groups (p=0.177) (Fig. 3).
The number of BrdU-positive cells in all three regions were similar across all groups (p=0.430, p=0.393, and p=0.106 for the IC, BZ, and CH, respectively).
The OD of Ang1 and synaptophysin in the IC was significantly higher in the low-frequency group than in the sham group (p=0.03 and p=0.03, respectively) (Fig. 4).
The level of Akt phosphorylation in the IC was increased in the low-frequency group. Akt phosphorylation in the BZ was significantly higher in the low-frequency stimulation group than in the high-frequency stimulation group (Fig. 5A).
The level of eNOS phosphorylation in the stimulated cortex was significantly higher in the low-frequency stimulation group than in the high-frequency stimulation group (Fig. 5B). Moreover, eNOS phosphorylation in the CH was significantly higher in the low-frequency group than in the sham and high-frequency stimulation groups (Fig. 5B).
In real-time PCR, the low-frequency group demonstrated signs of significantly higher levels of TEK transcription in the IC than the sham stimulation group (p<0.05) (Fig. 6).
In this study, rTMS on the lesioned hemisphere after stroke induced changes in the angiogenic mechanisms. The expression of angiopoietin 1 and synaptophysin in the IC was significantly higher in the low-frequency group than in the sham stimulation group. Angiopoietin 1 has a pivotal role in the regulation of physiological angiogenesis. It has also been linked with improved neurological function after stroke in a recent study on rats [27]. Synaptophysin is a membrane protein of the synaptic vesicle and is useful as a marker of synaptogenesis and axonal sprouting [28]. Low-frequency rTMS on the IC may stimulate angiogenesis and synaptogenesis and thus promote functional recovery after stroke.
Akt phosphorylation was significantly increased in the BZ with low-frequency stimulation than with high-frequency stimulation. There was no significant difference between low-frequency stimulation and sham stimulation, though the Akt phosphorylation levels were higher in the low-frequency stimulation group. Increased Akt kinase activity contributes to cell survival and angiogenesis [29]. Our results suggest that low- and high-frequency stimulations may have opposite effects with low frequency increasing and high frequency decreasing Akt phosphorylation. Interestingly, Akt phosphorylation was significantly increased only in the BZ. The peri-infarct cortex is a site for many endogenous recovery processes [30]. Different levels of recovery process activities may have been the reason for different effects of rTMS. eNOS phosphorylation was increased significantly by the 2-week application of low-frequency rTMS compared with that achieved by high-frequency stimulation in all three sites. eNOS phosphorylation enhances angiogenesis [31]. Again, our results suggest that high-and low-frequency stimulation may have opposite effects on the angiogenic mechanisms. Different stimulation frequencies may have differing effects on the angiogenic mechanisms after stroke. Another possible explanation is that the changes caused by high-frequency rTMS have a higher threshold or require a longer duration than those by the low-frequency rTMS. eNOS phosphorylation was significantly increased with low-frequency stimulation in the CH than that with sham stimulation. This may be the mechanism through which low-frequency rTMS at the contralateral side promotes functional recovery after stroke.
The genetic expression of TEK gene, mainly expressed in the endothelial cells of blood vessels, was significantly increased in the IC in the low-frequency stimulation group than that in the sham stimulation group. Our results show that low-frequency rTMS can modulate the expression of genes involved in angiogenesis in the IC. Previous studies have reported the effect of rTMS on genetic expression [14,15]. Mice treated with rTMS for up to 40 days showed alteration in glutamate and glycine transporter mRNAs. Multiple endoplasmic reticulum stress-related genes were also shown to be altered [14]. A previous study on rats reported upregulation of genes related to angiogenesis (BAI1 and VEGFA) after rTMS [15]. Our study results extended these findings, demonstrating that rTMS affects angiogenesis-related genes.
Some limitations need to be considered when interpreting our study results. First, the number of rats analyzed for immunohistochemistry, Western blot, and RT-PCR was small. Correlation analysis between increased angiogenesis and improved functional outcome could not be performed. While angiogenesis is an integral part of neural recovery, the clinical significance of increased angiogenesis after rTMS is unclear and is yet to be determined. Second, only two stimulation frequencies, 1 Hz and 20 Hz, at the ipsilateral side were examined. The optimal stimulation protocol for recovery needs to be further elucidated. Third, the rats were divided into mild and severe groups based on the behavioral tests, to minimize bias due to the severity of ischemia. Diffusion-weighted magnetic resonance imaging or laser Doppler was not performed because of time and cost limitations. Lastly, the rats were sacrificed at day 17 after stroke. The study was unable to establish whether the changes were long lasting, and the extent to which changes in gene expression levels led to actual functional recovery.
In summary, low-frequency rTMS of the lesioned hemisphere in the early subacute phase following a stroke promotes the expression of angiogenic factors and related genes in the brain, particularly in the IC region. In addition to the well-known effects of rTMS, the regulation of Tie2, Akt, and eNOS appears to play vital roles in rTMS-induced changes. Additional studies are needed to determine the optimal stimulation parameters.
ACKNOWLEDGMENTS
This work was supported by a grant from Seoul National University Hospital Research Fund (No. 04-2010-0860), a Korea Research Foundation Grant funded by the Korean government (No. KRF-2008-313-E00458), and a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A091281). This study is a part of Dr. Byung-Mo Oh’s PhD thesis at Seoul National University College of Medicine.
AUTHOR CONTRIBUTION
Conceptualization: Oh BM, Han TR. Methodology: Oh BM, Park SH, Han TR. Formal analysis: Lee Y, Oh BM, Park SH. Funding acquisition: Oh BM, Han TR. Project administration: Oh BM. Visualization: Lee Y, Oh BM, Park SH, Han TR. Writing – original draft: Oh BM, Lee Y. Writing – review and editing: Lee Y, Oh BM, Park SH, Han TR. Approval of final manuscript: all authors.
Fig. 1
Experimental design. MCAO, middle cerebral artery occlusion; rTMS, repetitive transcranial magnetic stimulation; PCR, polymerase chain reaction.
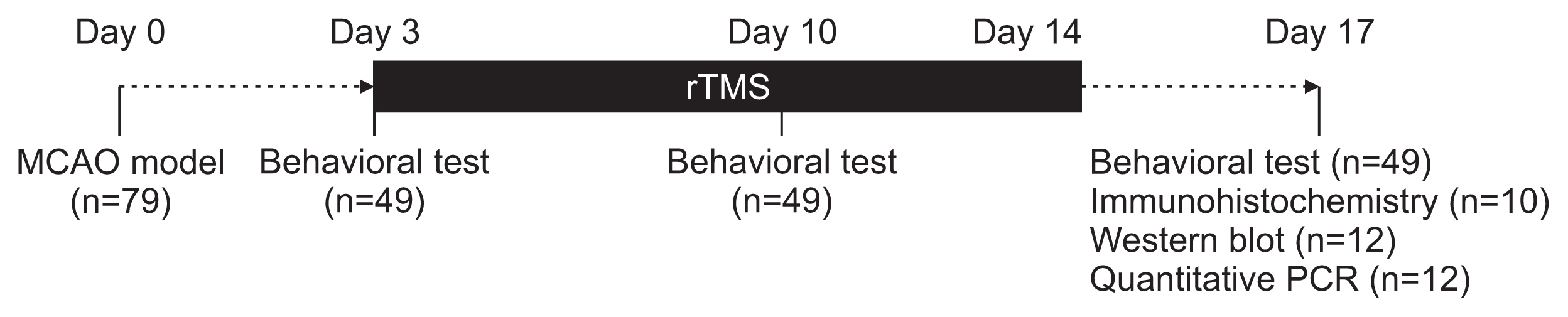
Fig. 2
Neurological function tests. There were no significant differences in (A) Garcia’s motor behavior score, (B) Rotarod test, and (C) modified foot fault test across the treatment groups at days 3, 10, or 17. Error bars represent the standard error of the mean.
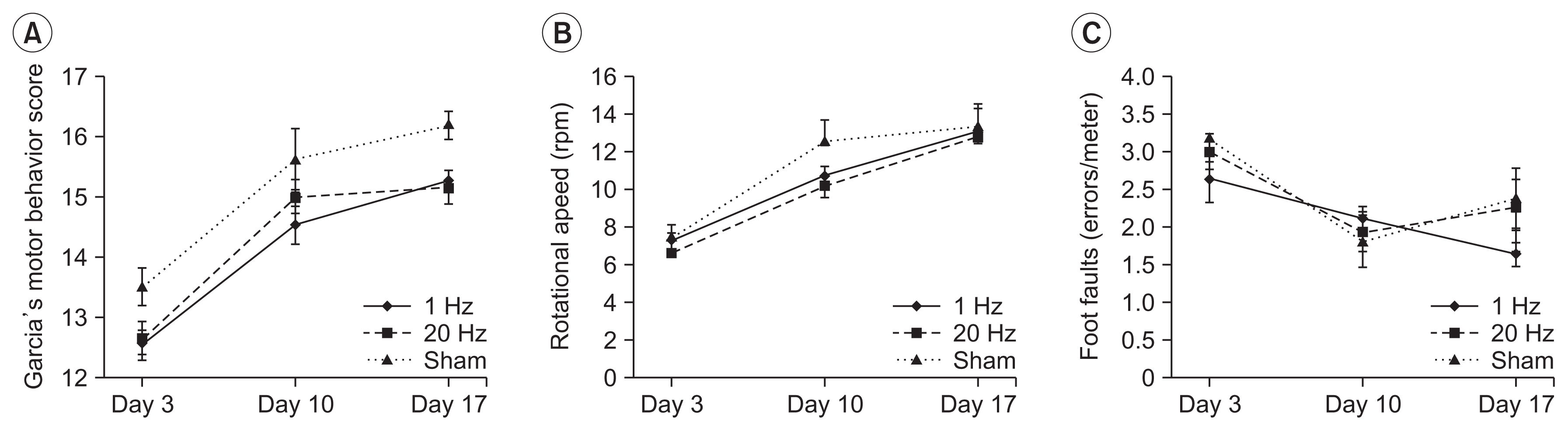
Fig. 3
Estimation of the infarct volume. Representative hematoxylin- and eosin-stained coronal section of the brain tissue are provided for (A) the sham, (B) 20 Hz, and (C) 1 Hz groups. The calculated volumes were similar across all three groups.
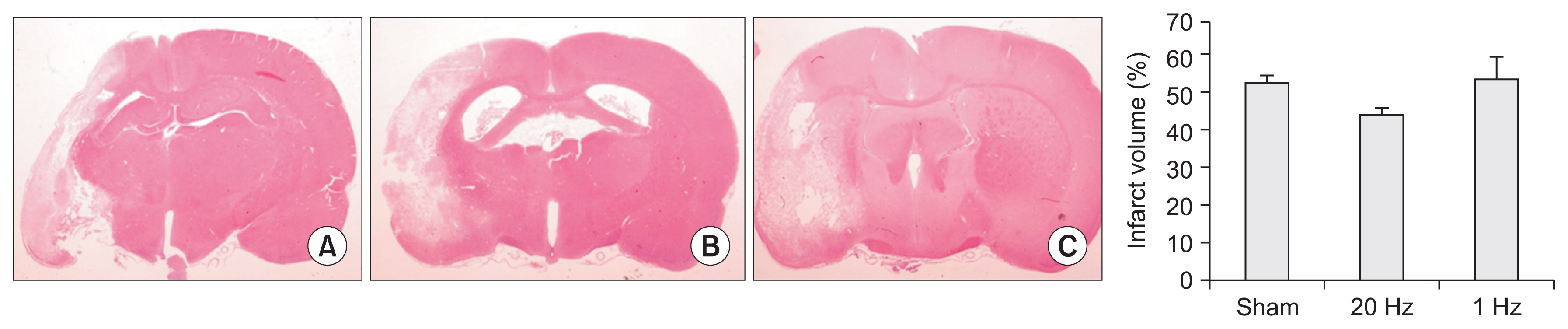
Fig. 4
Optical densities of (A) angiopoietin 1 and (B) synaptophysin in the ischemic core, border zone, and contralateral homologous cortices. *p<0.05.

Fig. 5
Results of Western blotting on phospho-Akt and phospho-eNOS. (A) Akt phosphorylation was significantly increased in the border zone in the low-frequency stimulation group compared to the high-frequency stimulation group. (B) Note the enhanced eNOS phosphorylation in the stimulated cortex with the low-frequency stimulation compared to the high-frequency stimulation (left). Interestingly, the Western blot demonstrates significantly higher levels of eNOS phosphorylation in the low-frequency group than in the sham and high-frequency stimulation groups in the contralateral cortex (right). *p<0.01.
Fig. 6
Expression of VEGFA, ANGPT1, and TEK in the ischemic core. Expression of TEK was significantly increased in the low-frequency stimulation group than that in the sham stimulation group. *p<0.05.
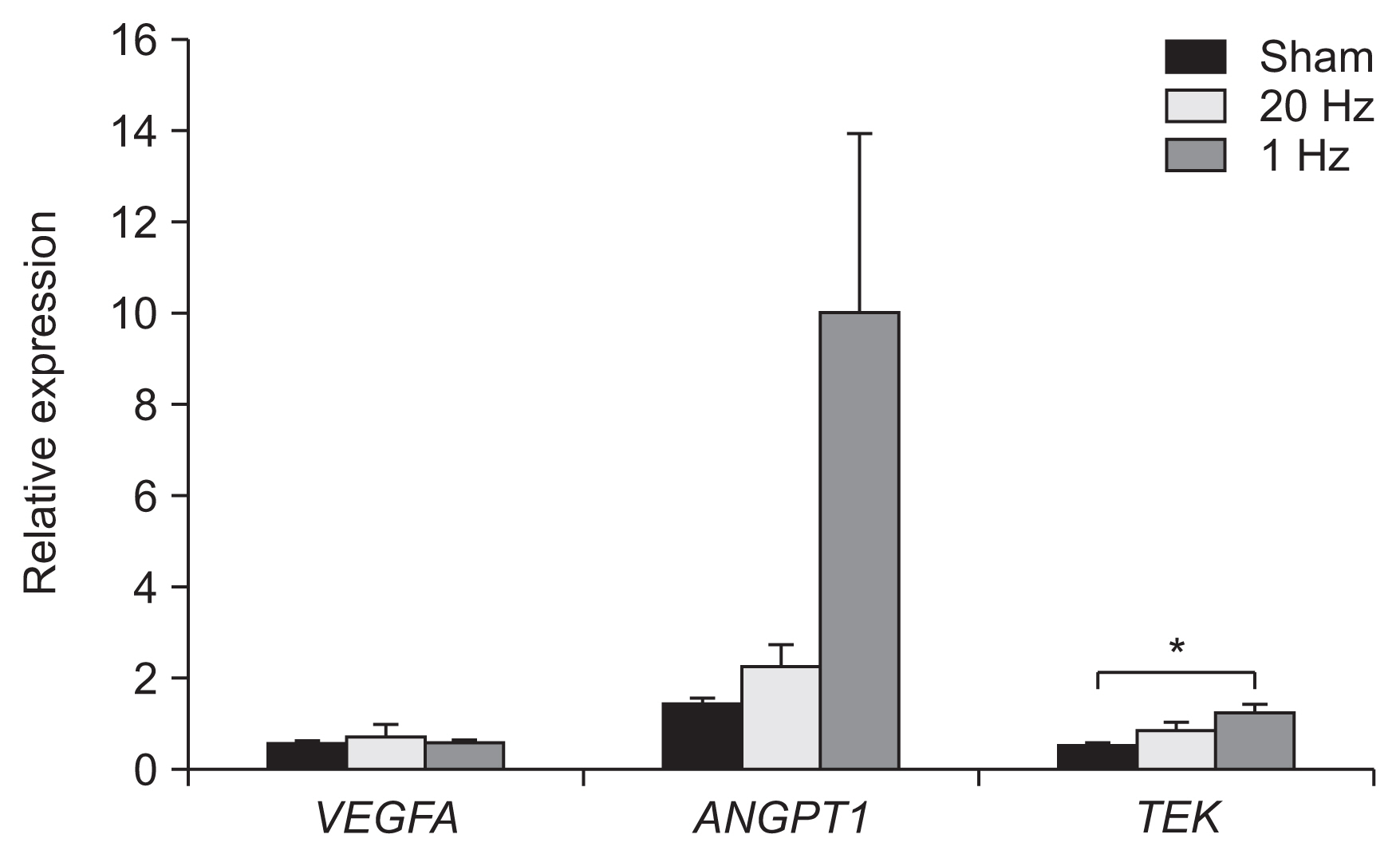
Table 1
Neurological functional tests
REFERENCES
1. Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry 2004;75:612-6.



2. Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain 2006;122:22-7.


3. O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007;62:1208-16.


4. Brighina F, Giglia G, Scalia S, Francolini M, Palermo A, Fierro B. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. Exp Brain Res 2005;161:34-8.



5. Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS). Neurophysiol Clin 2006;36:105-15.


6. Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology 2005;65:466-8.


7. Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 2006;37:2115-22.


8. Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994;117(Pt 4): 847-58.


9. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 2007;8:559-67.



10. Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res 2007;176:603-15.



11. Keck ME, Sillaber I, Ebner K, Welt T, Toschi N, Kaehler ST, et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur J Neurosci 2000;12:3713-20.


12. Post A, Muller MB, Engelmann M, Keck ME. Repetitive transcranial magnetic stimulation in rats: evidence for a neuroprotective effect in vitro and in vivo. Eur J Neurosci 1999;11:3247-54.


13. Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science 2007;317:1918-21.


14. Ikeda T, Kobayashi S, Morimoto C. Effects of repetitive transcranial magnetic stimulation on ER stressrelated genes and glutamate, γ-aminobutyric acid and glycine transporter genes in mouse brain. Biochem Biophys Rep 2018;17:10-6.



15. Ljubisavljevic MR, Javid A, Oommen J, Parekh K, Nagelkerke N, Shehab S, et al. The effects of different repetitive transcranial magnetic stimulation (rTMS) protocols on cortical gene expression in a rat model of cerebral ischemic-reperfusion injury. PLoS One 2015;10:e0139892.



16. Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab 2002;22:379-92.



17. Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke 2012;43:2270-4.



18. Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab 2007;27:564-74.



19. Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 2000;156:965-76.



20. Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke 2001;32:2179-84.


21. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994;25:1794-8.


22. Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol 2007;62:49-58.


23. Caglayan AB, Beker MC, Caglayan B, Yalcin E, Caglayan A, Yulug B, et al. Acute and post-acute neuromodulation induces stroke recovery by promoting survival signaling, neurogenesis, and pyramidal tract plasticity. Front Cell Neurosci 2019;13:144.



24. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84-91.


25. Beom J, Lee JC, Paeng JC, Han TR, Bang MS, Oh BM. Repetitive transcranial magnetic stimulation to the unilateral hemisphere of rat brain. J Vis Exp 2016;(116): 54217.



26. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke 1995;26:627-35.


27. Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 2006;98:1014-23.



28. Seo HG, Kim DY, Park HW, Lee SU, Park SH. Early motor balance and coordination training increased synaptophysin in subcortical regions of the ischemic rat brain. J Korean Med Sci 2010;25:1638-45.



29. Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle 2006;5:512-8.










