A Fully Immersive Virtual Reality Method for Upper Limb Rehabilitation in Spinal Cord Injury
Article information
Abstract
Objective
To determine whether a fully immersive virtual reality (VR) intervention combined with conventional rehabilitation (CR) can improve upper limb function more than CR alone in patients with spinal cord injury (SCI), we conducted a prospective, randomized, controlled clinical trial.
Methods
Participants were randomly assigned to either the control group (CG; n=10) or experimental group (EG; n=10). The participants in the CG received 60 minutes of conventional therapy per day, 4 days per week for 4 weeks, whereas those in the EG received 30 minutes of VR training and 30 minutes of conventional therapy per day, 4 days per week for 4 weeks. The clinical outcome measures included Medical Research Council grade, the American Spinal Injury Association upper extremity motor score (ASIA-UEMS), and scores in the Hand Strength Test, Box and Block Test, Nine-Hole Peg Test, Action Research Arm Test, and Korean version of the Spinal Cord Independence Measure (K-SCIM). The assessments were performed at the beginning (T0) and end of the intervention (T1).
Results
Grip power and K-SCIM score significantly improved in the EG after the intervention. When comparing differences between the groups, elbow extensor, wrist extensor, ASIA-UEMS, grip power, lateral pinch power, and palmar pinch power were all significantly improved.
Conclusion
VR training of upper limb function after SCI can provide an acceptable adjunctive rehabilitation method without significant adverse effects.
INTRODUCTION
Spinal cord injury (SCI) causes motor and sensory deficits below the damaged level, reducing patient quality of life [1]. Depending on the level of injury, SCI can lead to serious complications, including autonomic dysreflexia, spasticity, respiratory system impairment, disturbances of the urinary and gastrointestinal systems, and sexual dysfunction.
The prevalence of SCI worldwide ranges from 10 to 100 cases per million individuals [2]. Approximately 60% of spinal cord-related injuries are cervical spinal cord injuries [3], which often involve severe losses of arm and hand functions and diminish life satisfaction [4]. As upper limb function plays a key role in independent daily activities such as self-management, respiration and sphincter management, and mobility, improving the upper extremity function is an important goal in rehabilitation [5].
The conventional rehabilitation (CR) is known to improve upper limb function [6], but providing sufficient treatment is labor intensive and expensive [7]. In addition, the traditional rehabilitation methods involving simple, repetitive movements may be tedious for the patient, making them less motivated to continue the treatment [8,9]. Therefore, new approaches must be introduced to overcome the shortcomings of the CR methods. One of the potential approaches is rehabilitation using virtual reality (VR) technology.
VR can be classified into non-immersive, semi-immersive, and fully immersive VR according to the user’s immersion level. Non-immersive VR systems have been used for many years in rehabilitation therapy for the purpose of cognition, gait, and balance training, including upper extremity motor function. Many previous studies in stroke patients have reported that VR is useful for rehabilitation [10] and can achieve greater improvements than the conventional therapies [11].
In contrast to non-immersive VR systems, in which users experience both the real world and virtual environment, immersive VR systems integrate users into an environment in which all real-world perception is blocked, so only computer-generated images are seen. Head-mounted display (HMD) devices are typically used to deliver a fully immersive VR experience. HMD devices can block the perception of the external environment to create a more realistic VR environment with a high level of immersion and visual scenes based on the user’s movements. As immersion increases, the emotional impact of VR on the user increases, and the desired physiological response can be driven through the production of the VR experience [12]. VR can be used to motivate patients by offering various realistic sensory experiences and entertainment-based treatments [10,13]. Furthermore, VR can be used as a practical technology to encourage patients to exercise regularly and simulate physical movements involved in everyday life for the purpose of improving patients’ capacity to cope with real-world situations [10,11].
Although many VR systems have been used in stroke patients and have produced promising preliminary results [8,10], few studies have examined their usefulness in patients with SCI. Thus, the purpose of the present study was to determine whether a fully immersive VR intervention combined with CR can improve upper limb function more than CR alone in patients with SCI.
MATERIALS AND METHODS
Participants
From March 2019 to July 2019, we prospectively enrolled 20 patients with upper limb dysfunction who were admitted at Chungnam National University Hospital in Daejeon, Korea. The inclusion criteria were as follows: (1) age between 19 and 75 years; (2) incomplete motor paralysis due to SCI at the C4–C8 neurological level; (3) <12 months since the onset of injury; (4) sufficient hand grip power to hold the controller; and (5) sufficient cognitive function to allow cooperation. The exclusion criteria were as follows: (1) inability to undertake the program due to pain; (2) severe spasticity (Modified Ashworth Scale grades 3 and 4); and (3) inability to sit alone. The patients were randomly assigned into two groups by using a random number table as follows: the experimental group (EG; n=10) experienced fully immersive VR combined with CR, and the control group (CG; n=10) experienced CR alone. This study was approved by the Institutional Review Board of Chungnam National University Hospital (No. 2019-01-055) and was registered in the Clinical Research Information Service (Registration No. KCT0003786). Written informed consents were obtained.
Study design
A prospective, randomized, controlled clinical trial was conducted. The participants in the CG received 60 minutes of conventional occupational therapy (OT) per day, 4 days per week, for 4 weeks. The patients assigned to the CG received 30 minutes of OT by a therapist, including shoulder, elbow, wrist, and finger joint exercises; hand grasping-release tasks; upper extremity strengthening; and stretching, and 30 minutes of activities-of-daily-living (ADL) training. By contrast, the participants in the EG received 30 minutes of VR training instead of ADL training and 30 minutes of OT per day, 4 days per week, for 4 weeks. The details are discussed in the VR training section.
VR training
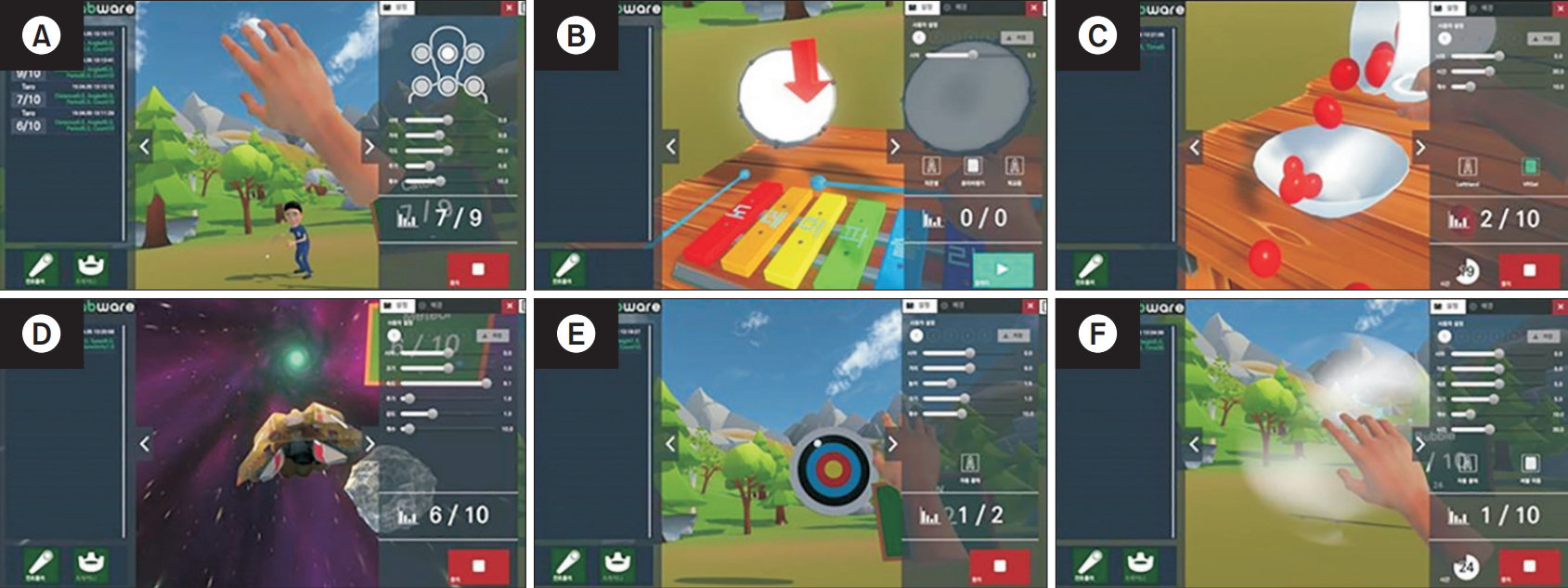
The immersive VR device used in this study, HTC VIVE VR RehabWare (Tech Village Corp., Goyang, Korea), delivers realistic movements in the virtual world through precision controllers, HMD headsets, vivid and realistic graphics, and feedback scores during the game (Fig. 1). This device tracks user movements with trackers and base stations to interact with the virtual world. Unlike stroke patients, patients with SCI typically have reduced upper extremity functions on both sides. As this device involves two controllers, it is useful for training both upper extremities. During VR training, the patient sat in the same chair with a backrest and performed six tasks (catching balls, playing xylophones, moving cherry tomatoes into a bowl, avoiding stones, throwing objects toward a target, and popping bubbles) using both hands, as shown in Fig. 2. Patients who could not remain seated were excluded, but an experienced occupational therapist always accompanied the patient as a precautionary measure to prevent harmful situations such as falling.
Assessment
The assessments were performed before the start (T0) and immediately after 4 weeks of training. Clinical outcome measures included Medical Research Council (MRC) grade, the American Spinal Injury Association upper extremity motor score (ASIA-UEMS), and scores in the Hand Strength Test (grip power, tip pinch power, lateral pinch power, and palmar pinch power), Box and Block Test (BBT), Nine-Hole Peg Test, Action Research Arm Test (ARAT), and Korean version of the Spinal Cord Independence Measure (K-SCIM).
Arm and hand muscle strengths
To test arm and hand muscle strengths, selected muscles (C5–T1: biceps, triceps, wrist extensor, finger flexor, and finger abductor) in both upper limbs were evaluated using the MRC grade (0=absent, 5=normal) in accordance with ASIA guidelines. In addition, the total scores for each limb (ASIA-UEMS) were used in the analysis. To test for grip and pinch strengths, a handheld dynamometer (BL5001 hydraulic hand dynamometer; B&L Engineering, Santa Ana, CA, USA) and pinch gauge (Saehan SH5005 hydraulic pinch gauge; Saehan Inc., Goyang, Korea) were used. The scores in three attempts were averaged for each hand.
Hand function
For distal measurement of upper limb function, we used the BBT and Nine-Hole Peg Test, which are commonly used to quantify hand function. The 19-item ARAT was used to measure hand function in the proximal limb, by grasping, gripping, pinching, and gross movement, rated on a 4-point scale from 0 (no movement) to 3 (movement performed normally).
Independence in ADL
The total K-SCIM score was measured on a scale from 0 to 100, including the following areas of function: selfcare, respiration and sphincter management, and mobility.
Statistical analysis
All data were analyzed using the SPSS version 22.0 statistical software (IBM, Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation, and categorical variables were expressed as a count (%). Baseline descriptive statistics were compared using the Mann-Whitney U-test for continuous data and the chi-square test for categorical data. The Wilcoxon signed-rank test was used for comparison within groups both at baseline and after treatment. The Mann-Whitney U-test was used to compare differences in values between the groups before and after intervention. For all the analyses, the statistical significance was set at p<0.05.
RESULTS
Table 1 shows the participants’ baseline characteristics. The study included 10 EG participants (6 males and 4 females) and 10 CG participants (8 males and 2 females) with motor-incomplete cervical SCI. No significant differences in baseline characteristics were observed between the two groups. Both upper extremities were evaluated separately, expressing the results as mean values, but no statistical differences were found. None of the study participants experienced an adverse event due to interaction with the virtual environment (e.g., nausea, dizziness, and headache) or falls, and none of the participants dropped out of the study.
Arm and hand muscle strengths
All selective muscle strength measures were improved after intervention in both groups, but no significant differences were observed (Table 2). When comparing the differences between the groups, elbow extensor, wrist extensor, and ASIA-UEMS were significantly improved.
Grip and pinch power
Both groups exhibited improvement after intervention, but only the increases in grip power were significant (p=0.02) (Table 2). Significant increases in grip power, lateral pinch power, and palmar pinch power were observed in the EG participants as compared with the CG participants (p=0.02, p=0.02, and p=0.04, respectively).
Hand function
We found no significant differences in the hand function test scores, except for the marginally significant improvements in the BBT scores after VR treatment (p=0.06).
Independence in ADL
Both groups exhibited significant improvements in total K-SCIM score, with no significant between-group differences (Table 2).
DISCUSSION
The goal of this study was to evaluate improvements of arm and hand functions and to assess the usefulness of a VR treatment program for cervical SCI as an adjunctive treatment modality for upper limb rehabilitation. The study was conducted with 16 sessions over 4 weeks, and we compared the results of VR treatment combined with CR in in the EG and those of CR alone in the CG.
This study demonstrated that VR training combined with CR resulted in functional improvement, particularly in grip power and K-SCIM score (Table 2). In addition, elbow extensor, wrist extensor, ASIA-UEMS, grip power, lateral pinch power, and palmar pinch power were all significantly improved as compared with those in the CG. Few studies have examined the VR methods in patients with SCI, and the findings of this study were inconsistent with previous studies. Dimbwadyo-Terrer et al. [14] reported that VR combined with CR showed similar results with CR alone in patients with tetraplegia. Unlike our study, that of Dimbwadyo-Terrer et al. [14] only targeted patients with motor-complete SCI (ASIA-A and ASIA-B), of which ASIA-A accounted for 11 (69%) of the 16 participants in the EG and 10 (67%) of the 15 in the CG. The participants in the EG played an ADL-based VR game to improve performance in basic ADL (eating, combing hair, or washing the face) instead of using controllers but freely moved to manipulate virtual objects. Another study by Prasad et al. [15] in 2018 also reported no significant difference in hand function improvement between the two groups. The participants in the EG used a controller in this study, but the study included both subjects with motor-complete and incomplete SCI, with the sum of ASIA-A and ASIA-B accounting for 7 (58%) of the 12 participants in the EG and 10 participants (70%) in the CG, respectively. In addition, the time from SCI onset to enrollment in the present study was about a month, while those in the studies by Dimbwadyo-Terre et al. [14] and Prasad et al. [15] ranged from 4 to 5 months and from 10 to 15 months, respectively. Previous research has shown that the potential for neurological recovery was greater in incomplete SCI than in complete SCI [16], motor recovery decreases 6 months after injury [17,18], early rehabilitation was effective, and the possibility of motor recovery was higher [19]. The discrepancy of findings between this study and the previous studies might be due to the fact that this study performed early rehabilitation for patients with motor-incomplete SCI who had been injured 1 month before or after rehabilitation.
However, previous studies in stroke patients showed consistent results. In a meta-analysis by Laver et al. [20], training with VR in stroke patients was found to improve ADL, with a moderate effect size. In another systematic review and meta-analysis, Lee et al. [21] reported that VR therapy improved muscle strength and ADL function in chronic stroke patients. Several recent studies have reported that rehabilitation using VR helped stroke patients improve their gross motor function [22]. Furthermore, several previous studies reported that VR training can be more effective and intensive than the existing treatments for improving functions in patients with damaged upper extremities due to stroke [23].
We observed functional improvements in both groups, but the EG participants in the present study may have exhibited improved hand strength because they exercised with controllers during the 30-minute intervention period, consistent with a previous study by Oh et al. [24], who reported improved hand strength after a VR intervention using a controller. Besides, the participant sees the results in the form of a score and receives positive feedback. Participants can develop advanced strategies for earning scores while viewing feedback scores during VR therapy [25]. This not only interests subjects but also triggers motor learning process.
Although improvements in upper limb function have been observed after VR treatment with CR in stroke patients, the evidence that applies to tetraplegia is still limited. Therefore, we believe that the present trial provides new insights into the role of VR applications in improving upper extremity function.
However, the present study involved several limitations that should be considered. First, the study sample was comprised of patients with tetraplegia caused by incomplete SCI only. Many previous studies examined patients with different prognoses in incomplete and complete injuries [26-28]. Therefore, patients with complete SCI must be included in future studies to determine the effects of damage due to SCI on VR treatment. Second, this was a relatively short-term study consisting of 16 rehabilitation sessions over 4 weeks. Long-term studies should be undertaken to gain a deeper understanding of the underlying mechanisms, as a short intervention period could not confirm whether VR is more effective than the traditional treatment for upper extremity rehabilitation. Finally, the patient sample in the present study was relatively small. Future studies with larger samples will be needed to confirm the present findings.
Despite the limitations of the present study, our findings demonstrate that VR training of upper limb function after SCI can provide an acceptable adjunctive rehabilitation method without significant adverse effects. In the future, larger controlled studies will be necessary to further evaluate the tolerability, feasibility, and efficacy of VR training.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was financially supported by the Ministry of Trade, Industry, and Energy (MOTIE), Korea, under the Regional Industry-Based Organization Support Program (No. P0001940) supervised by the Korea Institute for Advancement of Technology.
Notes
Conceptualization: Lim DY, Ahn SY. Methodology: Lim DY, Ahn SY, Cho KH. Formal analysis: Hwang DM, Moon CW. Funding acquisition: Ahn SY. Project administration: Ahn SY. Visualization: Hwang DM, Moon CW. Writing - original draft: Lim DY. Writing - review and editing: Ahn SY. Approval of final manuscript: all authors.