INTRODUCTION
Lymphedema is a chronic condition caused by dysfunction of the lymphatic system, responsible for maintaining tissue fluid balance [
1]. This condition is characterized by subcutaneous accumulation of extracellular fluid, resulting from lymphatic vessel dysfunction and could lead to inflammation and fibrosis. Treatments for malignancies in cancers of the breast, uterus, ovary, and prostate are known to cause secondary lymphedema. Surgery and radiotherapy for these malignancies greatly increase the risk of lymphedema in the extremities [
2]. A previous study reported that 20% of cervical cancer patients presented with lower extremity lymphedema [
3]. In Koreans, 11.1% of ovarian cancer patients developed lower extremity lymphedema after treatment [
4].
Lymphedema limits a patient’s physical activity and increases the risk of psychosocial problems and clinical complications, including cellulitis [
5]. Gynecological cancer related lymphedema (GCRL), after pelvic lymph node dissection, has been specifically shown to decrease patient quality of life [
6]. Early diagnosis and treatment of lymphedema may prevent its progression and associated complications [
7]. Therefore, early diagnosis and treatment are necessary for GCRL. Several different modalities are used to evaluate lymphedema, including extremity circumference measurement, volume measurement, tissue tonometry, ultrasonography, lymphoscintigraphy, dual energy X-ray absorptiometry (DEXA), and bioimpedance analysis (BIA).
Among these modalities, BIA is preferred as it is simple, inexpensive, and non-invasive. Volume measurement alone, does not account for changes in muscle mass, and is an imperfect assessment of the interstitial fluid change. Interstitial fluid change is very important in evaluating lymphedema. BIA has the advantage of being a sensitive measure of interstitial fluid changes, and a previous study has reported that multiple frequency BIA (MFBIA) reliably detects early-stage upper extremity lymphedema with a sensitivity of 100% [
2]. Low frequency current usually passes through the extracellular fluid, suggesting analysis at 0 Hz is ideal for evaluating lymphedema [
8]. However, factors such as hydration status, medical conditions, environmental factors, and ethnicity, can influence BIA results; as such, BIA absolute values alone are insufficient to discern changes of extracellular volume [
9]. Therefore, a comparison of BIA values between affected and unaffected sides has been previously used to evaluate unilateral breast cancer related lymphedema (BCRL) [
10].
Many studies have evaluated unilateral BCRL using BIA. BCRL presentation is usually unilateral, and BIA is, thus, performed using a ratio of affected extremity to unaffected extremity. An earlier study suggested a cutoff value of the extracellular fluid volume for assessing BCRL [
11]. Another study suggested extracellular fluid volume ratio and single frequency BIA (SFBIA) before CDT, as a useful modality for predicting treatment outcomes [
12].
The protocols for gynecological cancer surgery differ from those for breast cancer surgery, in that they include bilateral pelvic lymph node dissection. GCRL may thus develop on both sides, and current BIA is limited to comparisons between affected and unaffected sides. Recently Hayes et al. [
13] suggested the BIA ratio of upper extremity to lower extremity for identifying cutoff values in lower extremity lymphedema in GCRL. However, research on BIA in the evaluation of GCRL remains scarce.
The purpose of this study is to investigate the feasibility of whether the BIA ratios of upper to lower extremities can reliably predict treatment outcomes for CDT in GCRL.
MATERIALS AND METHODS
Study design
This study was a retrospective study, conducted at a single lymphedema clinic, in patients who had gynecological cancer surgery from March 2015 to December 2018. All lower extremity lymphedema diagnoses were confirmed by the authors of this study. Before data was collected, approval for this study was obtained from Asan Medical Center Institutional Review Board (No. S2019-1328-0001).
Subjects
All patients developed lower extremity edema at least 1 month after surgery for gynecological cancer. Most patients were evaluated by D-dimer, lower extremity venography CT or Doppler ultrasonography to assess deep vein thrombosis (DVT), and lymphoscintigraphy to uncover potential secondary lymphedema before CDT. Lymphedema was diagnosed on the basis of clinical symptoms such as swelling, heaviness, tightness, and fatigue; lymphoscintigraphic findings compatible with secondary lymphedema, and at least a 2 cm difference in lower extremity circumference between affected and unaffected sides, regardless of subjective symptoms for unilateral lymphedema. Inclusion criteria for the study were: clinical diagnosis of unilateral or bilateral GCRL, 10-day course of CDT after the development of lymphedema, and BIA performed before commencing CDT and after the 10-day course of CDT. Exclusion criteria were the presence of comorbid conditions that could lead to lower extremity lymphedema such as current metastasis, cellulitis, DVT or incomplete medical records.
In order to identify whether BIA is a sensitive modality for early GCRL diagnosis, we then divided the patients based on the duration from symptom onset to first bioimpedance measurement, with the acute group representing duration <6 months and the chronic group duration >6 months. The duration cutoff point was chosen in accordance with a previous study [
14].
Lymphedema treatment
CDT was performed on patients for 30 minutes per day for 10 days. CDT included manual lymphatic drainage, compression bandaging, skin care, and exercise education. The same physical therapist performed CDT on all patients.
Lymphedema evaluation
We conducted BIA analyses of the upper and lower extremities using the Inbody S10 (InBody, Seoul, Korea) before and after a 10-day course of CDT, as each session was conducted after a 5-minute rest. We also measured the circumferences of the lower extremities at 20 and 10 cm above the knee (AK) and 10 cm below the knee (BK), pre- and post-CDT.
In general, reactance and resistance values can be calculated using impedance and phase angle, measured at each frequency in MFBIA, and these values can be used to calculate the expected impedance measured at 0 Hz (R0) [
15]. We calculated the R0 of the four extremities using MFBIA to evaluate GCRL in the study subjects. Previous studies have used the upper/lower extremity R0 ratio to diagnose lower extremity lymphedema [
13]. Thus, we calculated the upper/lower extremity R0 ratio (R0
U/L), using the upper extremity R0 values for the ipsilateral side. We investigated the relationship between R0
U/L before CDT (pre-treatment R0
U/L) of the affected side, and the changes to R0
U/L (ΔR0
U/L) and circumference values (ΔCircumference) pre- and post-CDT, in patients with unilateral or bilateral GCRL. As the change of ratio, rather than absolute change was used as an analytic tool, all changes were presented as differences between pre- and post-CDT results.
Subgroup analysis was also performed to compare the predictive capacity of BIA between the acute and chronic groups. The primary outcomes of this study were the differences in R0U/L and lower extremity circumferences preand post-CDT.
Statistical analyses
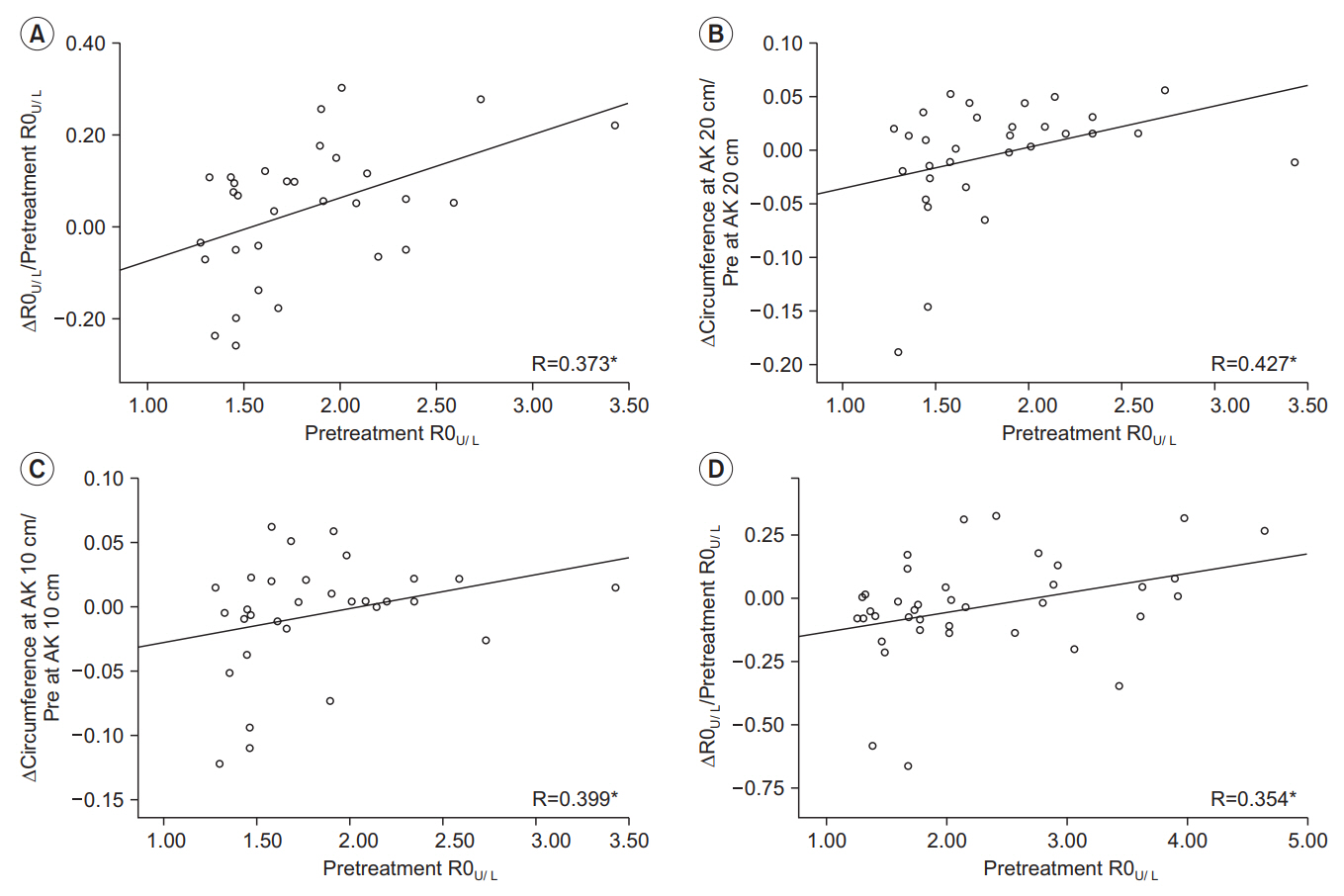
All statistical analyses were conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). In this study, we identified the differences between the acute and chronic groups, using an independent t-test for normally distributed data, and Mann-Whitney U-test for non-normally distributed data. The majority of data were not normally distributed, and thus we conducted Spearman correlation analysis to identify the relationships between pretreatment R0U/L of the affected side and ΔR0U/L, and ΔCircumference pre- and post-CDT. A partial correlation analysis was performed to make adjustments for age and body mass index (BMI) data that might have affected the results. A p-value of less than 0.05 was considered statistically significant.
DISCUSSION
Our results suggest that pre-treatment BIA can predict lower extremity circumference changes, reflecting volume changes after CDT, in the early stages of GCRL. To the best of our knowledge, this is the first study to clarify the relationship between pre-treatment BIA and volume change after CDT in the GCRL.
In this study, lymphedema was improved after CDT in most patients. However, some patients had no lymphedema improvement or got worse after CDT. CDT was performed on an outpatient basis, and the effects of CDT may have been different because of variability in patient compliance. This study investigated whether pre- and post-treatment BIA results reflect lymphedema changes pre- and post-CDT, rather than using BIA itself to diagnose lymphedema.
Previous studies have set specific diagnostic criteria for lower extremity lymphedema. In this study, the ratio of upper extremity R0 to lower extremity R0 was applied in such a way as to match against a cutoff value, used to diagnose lower extremity lymphedema. These values were >1.308 on the dominant side, and >1.340 on the nondominant side (sensitivity 75%, specificity 85%), as determined by a previous study [
13]. Other studies have also used these cutoff values to diagnose GCRL [
16]. Another study reported, that BIA was an appropriate method for the assessment of extracellular fluid in the lower extremities of healthy young people [
17]. Most studies, however, have focused on the diagnostic validity of BIA for lower extremity lymphedema.
In BCRL, a previous study evaluated the calculated extracellular fluid ratio of affected versus unaffected upper extremities with BIA and changes to upper extremity circumferences pre- and post-CDT. The result of that study suggested that pretreatment BIA values could predict treatment outcomes of CDT [
12].
In our study, we verified that pre-treatment BIA values could predict the treatment outcomes of CDT in GCRL. R0
U/L increased as lower extremity lymphedema worsened. As such, an increase in ΔR0
U/L pre- to post-CDT, indicated lymphedema improvement. Pre-treatment R0
U/L was positively correlated with ΔR0
U/L in both the acute and chronic groups, suggesting that pre-treatment R0
U/L was a reliable factor for predicting changes after CDT in GCRL. Furthermore, pre-treatment R0
U/L showed a tendency to be correlated with ΔCircumference in the acute group, although this correlation was not statistically significant after adjusting for age and BMI. These findings suggested, that lower extremity circumferences were further reduced after CDT, in patients with severe lymphedema in the acute group. These results indicated that pre-treatment R0
U/L held potential for predicting treatment outcomes for GCRL in the acute patients’ group. Therefore, we concluded that BIA predicted more reliably the effects of CDT for GCRL in the early stages of lymphedema, as opposed to the chronic stages. BIA might reflect accurately the extracellular fluid volume by calculating electric current flow through the body and is based on the principle that fat impedes electric current more than protein or fluid [
9]. In the chronic GCRL group (lymphedema duration ≥6 months), chronic inflammation and fibrosis may promote fat hypertrophy [
1], that could influence the BIA results in these patients. A lack of previous studies on the effects of different periods of duration of lymphedema on BIA results, limits the applicability of dividing the acute and chronic groups using a 6-month cutoff. Future studies, evaluating differences in BIA results according to lymphedema duration, will be necessary to explore this phenomenon in more depth.
Several limitations of this study must be acknowledged. First, the sample size was small and heterogeneous regarding cancer type, stage, and treatment. The correlations between pre-treatment R0U/Land ΔCircumference were not statistically significant, after adjusting for age and BMI (a potential effect of the small sample size). Further studies with larger populations were recommended. Second, while R0U/L represented the overall impedance measurement of the lower extremity, ΔCircumference might be less statistically significant because it only assessed changes to specific segments of the lower extremity. Further large-scale studies evaluating BIA for the segmental area of the lower extremity will be necessary. Third, unlike previous studies, the dominant extremity was not identified in this medical record review, preventing us from distinguishing between dominant and nondominant extremities, when calculating R0U/L. Finally, we could not control factors potentially affecting the results of BIA, such as temperature, hydration status, and physical activity just before measurement, all of which might have affected our results. While further studies are recommended, this study confirms the feasibility of BIA for predicting treatment outcomes of CDT in the early stages of GCRL.
In conclusion, findings from this study suggested that pre-treatment BIA values can predict interstitial fluid volume reductions after CDT in the early stages of GCRL. These findings implied that BIA parameters could be used to predict the treatment outcomes in the early stage of GCRL. We recommend further large-scale prospective studies to be conducted for more in-depth research.