The Value of MicroRNAs as an Indicator of the Severity and the Acute Phase of Spinal Cord Injury
Article information
Abstract
Objective
To assess the role of miRNA-21 and miRNA-223 in a balloon-compression model of spinal cord injury (SCI).
Methods
A total of 50 male Wistar rats (n=50) were divided into the three groups: the group A (n=15, insertion of the unflated Fogarty balloon catheter), the group B (n=15, insertion of the Fogarty balloon catheter at a volume of 20 μL) and the group C (n=15, insertion of the Fogarty balloon catheter at a volume of 50 μL). After the behavioral test, RNA isolation, microRNA expression profiling using microarrays and quantitative polymerase chain reaction, measurements were compared between the three groups.
Results
Despite a lack of significant differences in time-dependent changes in miRNA-21 expression levels between the three groups at 4 hours, there were significant differences in them at 1, 3, and 7 days (p<0.05). Moreover, there were significant differences in time-dependent changes in miRNA-223 expression levels between the three groups at 4 hours and 1, 3, and 7 days (p<0.05). Furthermore, miRNA-223 expression levels reached the highest at 1 day but were decreased with time thereafter in all the three groups.
Conclusion
Expression levels of miRNA-21 and miRNA-223 might be associated with the severity and acute phase of SCI, respectively. It is mandatory, however, to analyze changes in levels of inflammatory markers and the relevant biological pathways.
INTRODUCTION
Spinal cord injury (SCI) is a devastating event that arises from trauma to the vertebra; it is characterized by mechanical disruption of the spinal cord [1]. Current treatment modalities are effective only in a limited scope. Only acute methylprednisolone therapy has had protective effects on SCI [2]. The SCI is considered a serious health problem that may impair the quality of life in affected individuals [3]. It is therefore imperative that new therapeutic strategies be established for the treatment of patients with SCI for which its cellular and molecular pathophysiology should be further explored.
A better understanding of the pathophysiological mechanisms underlying the onset of SCI is essential for developing effective therapeutic approaches; its key events include injury and involvement of various factors resulting in the functional deficits. Thus, its pathophysiology is well described as a biphasic process; it consists of primary and secondary phase of injury. The primary phase of injury involves the initial mechanical impact that is characterized by exertion of the force to the spinal cord and the disruption of axons, blood vessels, and cell membranes. This is followed by the secondary phase of injury that is characterized by inflammation and delayed apoptotic events. Despite the immediate presence of neurological deficits following the onset of initial injury, the secondary phase of injury leads to a prolonged period of tissue destruction [4]. Thus, worsening of SCI is closely associated with its secondary pathophysiology, which leads to the extension of the paralysis to higher spinal segments. Patients with SCI are therefore vulnerable to paralysis due to inhibition of the generation of neuronal precursor cells after several years of persistence of such pathophysiological changes [5,6].
Over the past decade, many clinical and translational studies have been conducted to propose new treatment strategies. Ongoing studies focus on factors involved in the secondary pathophysiology of SCI, thus attempting to promote regeneration and replace destroyed spinal cord tissue [7].
Studies about complex interaction between the cellular and molecular pathophysiologic events of SCI have broadened the understanding of it. Next, inhibition of multiple pathogenic mechanisms and promotion of neuroregeneration should be further studied in this series. Moreover, the severity of SCI is known as a strong prognostic indicator that is closely associated with the neurologic grade on admission in patients with SCI [8].
MicroRNA (miRNA) sequences have a hairpin-like structure, and they are small, unique, non-coding RNA fragments with a mean length of 22 nucleotides. Their potential roles in regulating biological pathways underlying the pathophysiology of SCI have been described in the literature. Thus, they are involved in neurogenesis and cortical development [9].
To date, more than 550 miRNAs have been identified from mammalian cells and their biological roles have been well documented. Of these, several miRNAs such as miRNA-1, miRNA-10a, miRNA-338, miRNA-451, miRNA-34a, miRNA-133, miRNA-142-3p, miRNA-199, miRNA-10b and miRNA-219 are abundantly present in the spinal cord. This is accompanied by previous published studies showing that their up-regulation or down-regulation are associated with the onset of SCI [10-17]. Moreover, identical families of miRNAs might target the same categories of genes. It can therefore be inferred that miRNAs are concurrently involved in the regulation of specific physiological processes underlying the onset of SCI [15,16].
The SCI is characterized by two molecular events such as inflammatory responses and apoptotic events. First, the pathogenesis of both acute and chronic SCI is regulated by inflammatory responses that might play a key role in the onset of nerve injury and the control of regenerative responses. Second, apoptotic events of neurons and oligodendrocytes leading to impaired neuronal functions greatly contribute to the paralysis of patients with SCI [18]. From this context, both miRNA-21 and miRNA-223 are closely associated with the secondary pathophysiology of SCI; experimental studies have shown that they are involved in the apoptosis and the acute phase of inflammation [10,19].
We have therefore speculated that there would be dramatic improvements in the severity and symptoms of SCI if it becomes possible to clarify the roles of miRNA-21 and miRNA-223 in association with its onset.
Given the above background, we conducted this experimental study to assess the role of miRNA-21 and miRNA-223 in an animal model of SCI and to discuss their clinical and therapeutic implications. To do this, we monitored time-dependent changes in their expression levels. There is an emerging evidence that there are timedependent changes in RNA expression in the spinal cord and this may be critical in the progression of SCI [20,21].
MATERIALS AND METHODS
Ethics statement
The current study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (no. CR318049). All the laboratory procedures were performed in compliance with the revised guidelines of the US National Institutes of Health.
Experimental animals
For the current laboratory procedure, we housed 50 male Wistar rats (n=50), aged 8 weeks, weighing 300–320 g, in the animal facility with 12-hour light/dark cycles. The temperature of the facility was maintained at 25℃–28℃. The rats were allowed for free access to water and standard rat chow.
An animal model of SCI
We used a balloon-compression model of SCI as previously described [22]. The experimental rats were anesthetized using 3% isoflurane (Terrell; Piramal Critical Care Inc., Bethlehem, PA, USA). Then, the anesthesia was maintained with 2.5% isoflurane. The experimental rats were placed in sternal recumbency and the lumbosacral region was shaved accordingly. This was followed by the treatment with povidone and alcohol.
Of the experimental animals, 45 were randomized to three groups: group A (n=15, insertion of the unflated Fogarty balloon catheter), group B (n=15, insertion of the Fogarty balloon catheter at a volume of 20 μL), and group C (n=15, insertion of the Fogarty balloon catheter at a volume of 50 μL).
An animal model of SCI was established under the fluoroscopic guidance (MCA-6100 mobile C-arm system; Medison Xray Inc., Seoul, Korea) for which the experimental rats underwent insertion of the BD Perisafe 18 G×5” Weiss Epidural Needle with fixed wings, Modified Tuohy Point (BD, Franklin Lakes, NJ, USA) in the lumbosacral joint. This was followed by placement of a 2-F Fogarty balloon catheter (Edwards Fogarty; Edwards Lifesciences, Irvine, CA, USA) using the spinal needle in the epidural space. The 2-F Fogarty balloon catheter was filled with iohexol with saline diluted at a ratio of 1:1 (Omnipaque; Amersham Health, Cork, Ireland) and then connected to a 50-μL Hamilton syringe (type 1705; Hamilton Company, Reno, NV, USA). The tip of the 2-F Fogarty balloon catheter was placed in the 9th thoracic spine and then inflated to the volume of 20 and 50 μL for 10 minutes using iohexol with saline diluted at a ratio of 1:1. After confirming the location and shape of the balloon catheter, we deflated and then removed it. This was followed by urinary bladder maintenance at a frequency of two times/day without treatment with antibiotics.
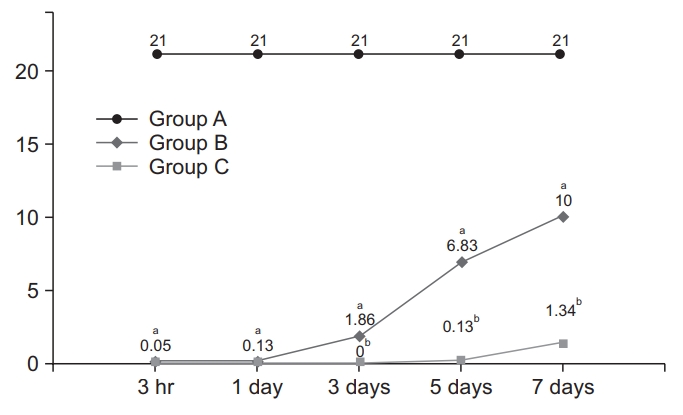
An animal model of SCI was validated on behavioral assessment and histopathological findings. Prior to sacrifice, gait and locomotor functions of the experimental rats were assessed using the Basso, Beattie and Bresnahan (BBB) locomotor scale, ranging from 0 (no locomotor activity) to 21 (normal function) as previously described [23]. Thus, the BBB scores were plotted as functions of the length of time (at 3 hours and 1, 3, 5, and 7 days).
RNA isolation
Total RNA was extracted from the parenchyma of the spinal cord using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and its concentration and quantity were measured based on the UV absorbance at a wavelength of 260 and 280 nm (A260/280) and then confirmed using a gel electrophoresis. The RNA sample was used for the quantitative polymerase chain reaction (PCR).
Quantitative PCR
For the quantitative PCR, we used a TaqMan miRNA assay kit (Applied Biosystems, Foster City, CA, USA). Then, we performed reverse transcription reactions for the mature miRNA containing a sample of total RNA, 50 nM stem-loop RT primer, 10× RT buffer, 100 mM of each dNTP, 50 U/μL MultiScribe Reverse Transcriptase and 20 U/μL RNase inhibitor. This was followed by incubation of reaction mixtures (15 μL) in a thermal cycler (Rotor-Gene Q; Qiagen, Hilden, Germany) for 30 minutes at 16℃, 30 minutes at 42℃, and 5 minutes at 85℃. Then, the reaction was maintained at 4℃.
The real-time PCR (RT-PCR) was performed using the Rotor-Gene Q instrument with a 10 μL of the PCR mixture containing a 1.33 μL of the RT-PCR product, 2× TaqMan Universal PCR Master Mix, 0.2 μM TaqMan probe, 15 μM forward primer, and 0.7 μM reverse primer. All the reaction mixtures were incubated in duplicate in a 72-well rotor at 95℃ for 10 minutes, followed by 40 cycles of 95℃ for 15 seconds and 60℃ for 1 minute. The U6 served as a control to normalize differences in total RNA levels between the samples. Following analysis of a threshold cycle (Ct) in the exponential phase of amplification, relative expression levels were quantified using standard curves for target genes and the endogenous control. Following calculation of the ΔΔ Ct values using geometric means, thus expressed as 2-ΔΔ Ct, the ΔΔ Ct value of each control sample was set at 1. Thus, changes in relative expression levels of target genes were analyzed.
Statistical analysis
For statistical analysis, all data was expressed as mean±standard deviation. Data analysis was performed using the SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences in measurements between the three experimental groups were tested for statistical significance using the repeated measures analysis of variance (ANOVA) and Duncan’s post-hoc analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
Validation of an animal model of SCI based on results of behavioral assessment
There were no time-dependent changes in the BBB scores in the group A. But the group B and C showed significant time-dependent changes in them (p<0.05) (Fig. 1).
Time-dependent changes in miRNA-21 expression levels
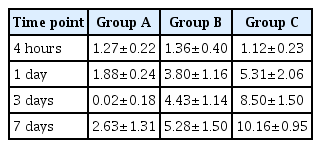
Time-dependent changes in miRNA-21 expression levels are shown in Table 1 and Fig. 2. At 4 hours, there were no significant differences in them between the three groups. At 1, 3, and 7 days, however, there were significant differences in them between the three groups (p<0.05). These results indicate that miRNA-21 expression levels might serve as an indicator of the severity of SCI.
Time-dependent changes in the level of miRNA-21 expression. Despite a lack of significant differences in time-dependent changes in miRNA-21 expression levels between the three groups at 4 hours, there were significant differences in them at 1, 3, and 7 days (p<0.05). Different letters indicate statistical significance (p<0.05). The y-axis represents 2-ΔΔCt (Ct, threshold cycle).
Time-dependent changes in miRNA-223 expression levels
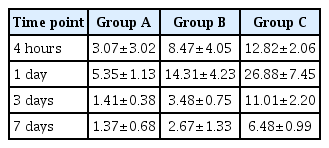
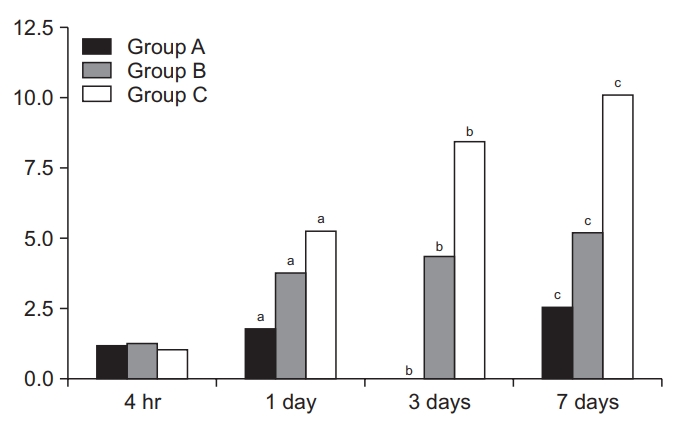
Time-dependent changes in miRNA-223 expression levels are shown in Table 2 and Fig. 3. At 4 hours and 1, 3, and 7 days, there were significant differences in them between the three groups (p<0.05). Moreover, miRNA-223 expression levels reached the highest at 1 day but were decreased with time thereafter in all the three groups. These results indicate that miRNA-223 expression levels might serve as an indicator of the acute phase of SCI.
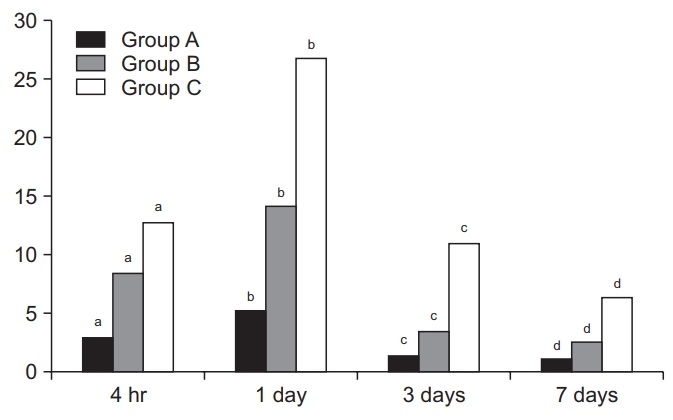
Time-dependent changes in the level of miRNA-223 expression. There were significant differences in time-dependent changes in miRNA-223 expression levels between the three groups at 4 hours and 1, 3, and 7 days (p<0.05). Moreover, miRNA-223 expression levels reached the highest at 1 day but were decreased with time thereafter in all the three groups. Different letters indicate statistical significance (p<0.05). The y-axis represents 2-ΔΔCt (Ct, threshold cycle).
DISCUSSION
To date, considerable efforts have been made to clarify the pathophysiology of SCI. This has contributed to the development of pharmacologic and cell-based therapeutic approaches, which is accompanied by animal models showing a functional motor recovery. Of these, several therapeutics have also been tested in clinical trials [24].
It is generally believed that SCI is an incurable disease leading to permanent disabilities. But there is a possibility that it might be treated with some therapeutic regimens as shown in studies about the efficacy of pharmacological agents and hypothermia [25]. Methylprednisolone is an effective neuroprotective agent, but its adverse effects remain a great concern. Hypothermia is also an effective neuroprotective modality, but its effectiveness deserves further evidence-based clinical studies. In addition, neuroregenerative approaches are also attempted for the treatment of SCI. They are advantageous in that they are improving endogenous regenerative potentials without adverse effects. Recent emergence of cell-based therapies has been of increasing interest as a neurotrophic support for the treatment of SCI [26].
Despite promising results of clinical studies in this series, there is still a lack of gold standard. It would therefore be mandatory to conduct future studies focusing on both neuroprotective and neuroregenerative approaches [27].
For the current experimental study, we used a ballooncompression model of SCI; we applied three different volumes to the rats and thereby successfully created an animal model of SCI with a severity ranging from a nearnormal condition to a severe one. This was demonstrated on behavioral test showing significant differences in BBB scores between the three groups. It can therefore be inferred that a balloon-compression model of SCI; its functional outcomes may have a correlation with the degree of stimulus applied to the rats [28].
In the current study, despite a lack of significant differences in time-dependent changes in miRNA-21 expression levels between the three groups at 4 hours, there were significant differences in them at 1, 3, and 7 days. These results indicate that miRNA-21 expression levels might serve as an indicator of the severity of SCI. Of note, up-regulation of miRNA-21 was associated with several types of CNS injuries, such as traumatic brain injury and brain ischemia [29,30]. This is accompanied by published studies showing that the dysregulation of miRNA-21 was involved in the pathogenesis of SCI in a rat SCI model [14,15]. Taken together, it can be inferred that miRNA-21 expression might be associated with the onset of SCI. Still, however, little is known about its association with the severity of SCI.
We also found that there were significant differences in time-dependent changes in miRNA-223 expression levels between the three groups at 4 hours and 1, 3, and 7 days. Moreover, miRNA-223 expression levels reached the highest at 1 day but were decreased with time thereafter in all the three groups. These results indicate that the level of miRNA-223 expression might be an indicator of the acute phase of SCI. This is in agreement with a previous report; Izumi et al. [19] showed that there was upregulation of miRNA-223 in the early phase of secondary damage after the onset of SCI.
Limitations of the current study are as follows. (1) We failed to analyze inflammatory markers that are indicative of the severity of SCI. To date, considerable efforts have been made to identify inflammatory markers in this series. Thus, it has been suggested that a series of inflammatory markers (IL-6, IL-8, and MCP-1) and structural proteins (tau, S100β and GFAP) are associated with the severity of SCI [31-33]. This is based on previous published studies showing that there is a correlation between levels of inflammatory cytokines after the onset of SCI and its severity [34,35]. (2) We failed to analyze biological pathways associated with the severity of SCI. According to Sengupt et al. [36], of the 49 proteins isolated from human cerebrospinal fluid samples, 8 showed a differential expression. These authors suggested that these proteins are involved in several molecular pathways underlying a set of pathophysiologic mechanisms depending on the severity of SCI in response to the assault. (3) There is a discrepancy in SCI between experimental models and a clinical settings. That is, animal models of SCI are characterized by damages to the thoracic spine and they occur dorsally. But human SCI occurs anteriorly and it is characterized by frequent damages to the cervical spine. Moreover, there is a close relationship between the location of lesions and the pathology and degree of SCI. That is, the anterior spinal artery and the dorsal one are affected in humans and animal models, respectively. The former is responsible for the blood supply for 3/4 of the spinal cord tissue [37,38]. It would therefore be mandatory to reflect the severity of SCI as closely as possible to clinical settings in establishing animal models of it.
In conclusion, expression levels of miRNA-21 and miRNA-223 might be associated with the severity and acute phase of SCI, respectively. But further studies are warranted to analyze changes in levels of inflammatory markers and the relevant biological pathways.
Notes
No potential conflict of interest relevant to this article was reported.
Notes
Conceptualization: Park J, Yi DS, Jang J, Hong J. Methodology: Park J. Formal analysis: Park J, Jang J. Project administration: Yi DS, Hong J. Visualization: Park J, Hong J. Writing - original draft: Park J, Yi DS. Writing - review and editing: Park J, Yi DS. Approval of final manuscript: all authors.