Transabdominal Functional Magnetic Stimulation for the Treatment of Constipation in Brain-Injured Patients: A Randomized Controlled Trial
Article information
Abstract
Objective
To investigate the effects of the transabdominal functional magnetic stimulation (A-FMS) for constipation in stroke or brain-injured patients.
Methods
Twenty-four brain-injured patients (11 males and 13 females; median age, 65 years; 22 cases of stroke and 2 cases of traumatic brain injury) with constipation, who were admitted to the rehabilitation department, were enrolled and randomly divided into magnetic stimulation (MS) group and sham stimulation (Sham) group. Several parameters related with constipation such as total and segmental colon transit time (CTT), defecation frequency, and Bristol Stool Scale (BSS) before and after 2 weeks of A-FMS (5 times per week, total 10 times of A-FMS) were evaluated. The Korean version of the Modified Barthel Index (K-MBI) was also evaluated.
Results
A significant decrease in segmental CTT in the left colon (-8.2±3.9 vs. 4.1±2.5 hours; p<0.05 by paired sample t-test) and a significant increase in the frequency of defecation (1.5±0.2 vs 0.7±0.3; p<0.05 by paired sample t-test) were observed in the MS group compared with the Sham group. Stool hardness became significantly softer in the MS group compared with the Sham group (2.3–3.5 in the MS and 2.6–3.1 in the Sham; p<0.05 by chi-square test) as evaluated by BSS. No difference in the K-MBI was observed between the two groups.
Conclusion
The present study suggests that A-FMS can be an additional therapeutic tool for managing constipation in brain-injured patients with abnormal bowel movement, defecation frequency, and stool hardness.
INTRODUCTION
Defecation is a basic physiological function in all people. In general, hard fecal consistency, decreased evacuation frequency, and sensation of incomplete evacuation are observed in 2%–27% of the general population [1,2]. In patients with stroke or traumatic brain injury, various factors of the brain-gut axis between neurological and gastrointestinal systems are impaired [3,4] of which constipation and fecal incontinence are the most common. In a systematic review, the incidence of constipation was noted as follows: 51%–66% in stroke patients, 45% in the acute stage of stroke, 48% during rehabilitation period [5], and 79.4% in stroke patients admitted to rehabilitation wards [6]. Constipation has a negative impact on social functioning and quality of life, leading to increased hospitalization, poor neurological outcome, increased incidence of complications, and death [7]. Currently, treatments for constipation after brain injury include dietary control, abdominal massage, bio-feedback, laxatives, enemas, and prokinetic agents as well as limiting drugs that lead to constipation [7]. Non-invasive functional magnetic stimulation (FMS) in combination with the abovementioned therapies promote bowel movement [8], and the positive effect of non-invasive electrical or magnetic stimulation on constipation has previously been reported [9-12]. Earlier, patients with spinal cord injury underwent either direct abdominal stimulation from the anterior abdomen (transabdominal) or via sacral FMS of the pelvic nerve at the posterior S2-4 [12]. Recently, transabdominal FMS (A-FMS) has been reported to reduce colon transit time (CTT), increase bowel movements, and reduce stool hardness in conditions of chronic constipation in stroke patients [13]. However, in the reported study, the comparison was made under stimulation and absence of stimulation in the same stroke patients and the number of subjects was limited to 12. Apparently, the present study was designed as a randomized controlled study to investigate the effect of A-FMS on constipation—movement of bowel, defecation frequency as well as fecal consistency—in brain-injured patients.
MATERIALS AND METHODS
Subjects
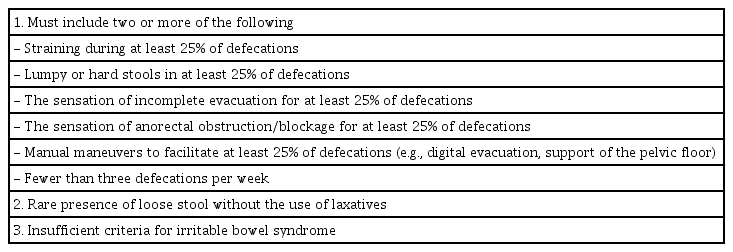
The brain-injured patients exhibiting no improvement in constipation by medication were admitted to the Department of Rehabilitation Medicine at Presbyterian Medical Center from July 2013 to March 2017. Thirty-one patients participated in the study but a total of 24 participants were enrolled. The subjects were included in the study based on the following inclusion criteria: (1) male/female adults aged 20 years or older diagnosed with stroke or traumatic brain injury on computerized tomography or magnetic resonance images and who had passed at least 2 weeks after stroke onset, (2) presence of stable vital signs, (3) those who consumed diet per oral or by Levin tube or by gastrostomy, and (4) those who met the criteria of functional constipation. Functional constipation was determined based on the Rome III criteria [14] (Table 1). The exclusion criteria included: (1) history of abdominal surgery, (2) past or current diagnosis of hypothyroidism, (3) small or large bowel problems—dysplasia of the anus, irritable bowel syndrome, and congenital megacolon, and (4) pregnant women.
The study was deemed appropriate and approved by the Institutional Review Board of Presbyterian Medical Center (No. 2013-01-04). The study was performed in accordance with the Declaration of Helsinki, and all the enrolled patients submitted a written informed consent for participation.
Clinical evaluation
Subjects were randomly assigned to either magnetic stimulation (MS) or sham stimulation (Sham) groups using a permuted block randomization. They continued taking the laxatives as they had been taking prior to enrollment and maintained their regimen throughout the study. During the first week, the CTT, the frequency of defecation per week, stool hardness, and the Korean version of the Modified Barthel Index (K-MBI) were evaluated. Same variables were re-evaluated during the 4th week after 2 weeks of A-FMS application. CTT was administered in capsule form (Kolomark, MITech, Pyeongtaek, Korea) containing 20 radiopacity markers at 9 a.m. for 3 consecutive days. Patients who were on tubefeeding had capsules directly inserted into the stomach via a gastroendoscope by a gastroenterologist. On the 4th day, supine simple abdomen radiography was taken and the radiograph was subdivided the colon into three segments: right colon, left colon, and rectosigmoid colon. The number of radiopacity markers present in each segment was counted and multiplied by 1.2 to calculate CTT (segmental CTT) and the total CTT was calculated by summation of its entirety [15] (Fig. 1). The frequency of defecation (times/week) was recorded. The stool hardness was classified into 1 through 7 types according to the shape and consistency using the Bristol Stool Scale (BSS) (Table 2) and the different types of stool hardness were converted into points. Investigators were aware of the group of each patient and magnetic stimulation was done by the therapists in the stimulation room, but the K-MBI, BSS, and frequency of defecation were evaluated blindly by each resident in-charge of the patients.

Simple abdomen radiographs show (A) three segments of the colon (right, left, and rectosigmoid colon) and distribution of radiopacity markers (arrows), and (B) before and (C) after 2 weeks of transabdominal functional magnetic stimulation. Three segments were divided with imaginary lines starting from the spinous process of a 5th lumbar vertebra to the upper spinous processes, to passing the right pelvic outlet, and to passing the left iliac crest. To calculate the colon transit time, the number of remaining radiopacity markers were counted and multiplied by 1.2 [15].
Transabdominal FMS application
At the 2nd week juncture, a circular coil magnetic stimulator (BioCon-1000Pro; Mcube Technology, Seoul, Korea) was applied on the segment in which radiopacity markers were retained the most as per supine simple abdomen radiograph on the 4th day of the first week. This stimulator generates a rapidly changing magnetic field, producing a brief burst (pulse width 370 μs) of very high current with a 1.5 Tesla (T) that is equivalent to 15,000 gauss (G), sufficient to stimulate the peripheral nerve and the intensity of the secondarily produced electrical field in nervous tissue is related to the rapidity of the change in magnetic field strength [16]. We chose 1.5 T intensity according to the previous reference study [12] to stimulate mainly the pelvic splanchnic nerve and to stimulate the abdominal muscles for increasing the abdominal pressure. The secondarily induced electrical strength was 10 V at 1 cm and 3 V at 5 cm from the stimulator head. The patients were treated with magnetic stimulation in a comfortable supine position (Fig. 2). A-FMS was administered for 20 minutes daily (3-second stimulation followed by 6-second rest) with 40 Hz frequency and intensity of 1.5 T (maximal intensity) for five times weekly for 2 weeks. In the Sham group (Fig. 2A), the intensity of 0.5 T (30% of maximal intensity) was applied at a distance 5 cm away from the abdomen to stimulate the abdomen while using the same criteria applied for other parameters in the MS group (Fig. 2B). The patient’s condition and the occurrence of any adverse events were monitored throughout the study period.
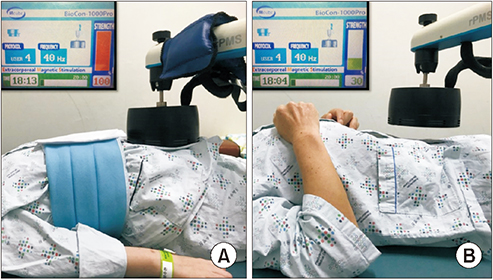
Transabdominal functional magnetic stimulation is applied in an experimental patient (A) and sham stimulation in control patient (B). In (B), note that the magnetic head is 5 cm apart from the patient’s belly and the stimulation intensity is 30% of the maximal intensity (1.5 T) so that the patient hears stimulation sound but does not feel the magnetic stimulation.
Statistical analysis
Data analysis was done by R language v3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). We used Student t-test for total and segmental CTT and K-MBI, Welch test and Fisher exact test for analyzing the frequency of defecation and chi-square test for assessment of fecal consistency to compare the differences in changes between the MS group and the Sham group. The level was considered significant when the p-value was less than 0.05.
RESULTS
General characteristics
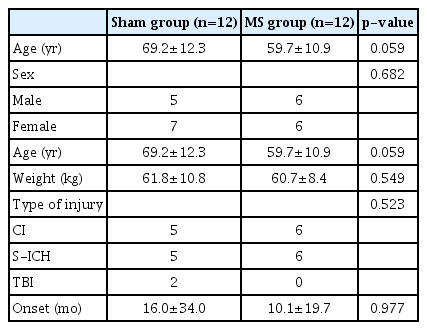
Of the 31 patients, 7 were dropped from the study due to discharge or transfer, resulting in a total of 24 participants (12 in the MS group and 12 in the Sham group). The mean age of the patients in the MS group was 59.7±10.9 years (6 males and 6 females; 6 cerebral infarction and 6 hemorrhage; disease duration, 10.1±19.7 months) and the mean age in the Sham group was 69.2±12.3 years (5 males and 7 females; 5 cerebral infarction, 5 spontaneous intracranial hemorrhage, and 2 traumatic brain injury; disease duration, 16±34 months) (Table 3). There was no difference in parameters between the two groups. The capsules containing the radiopacity markers were inserted into the stomach by a gastroendoscope in 1 patient in the MS group and in 1 patient in the Sham group while the remaining participants the route of administration was oral.
Changes in CTT
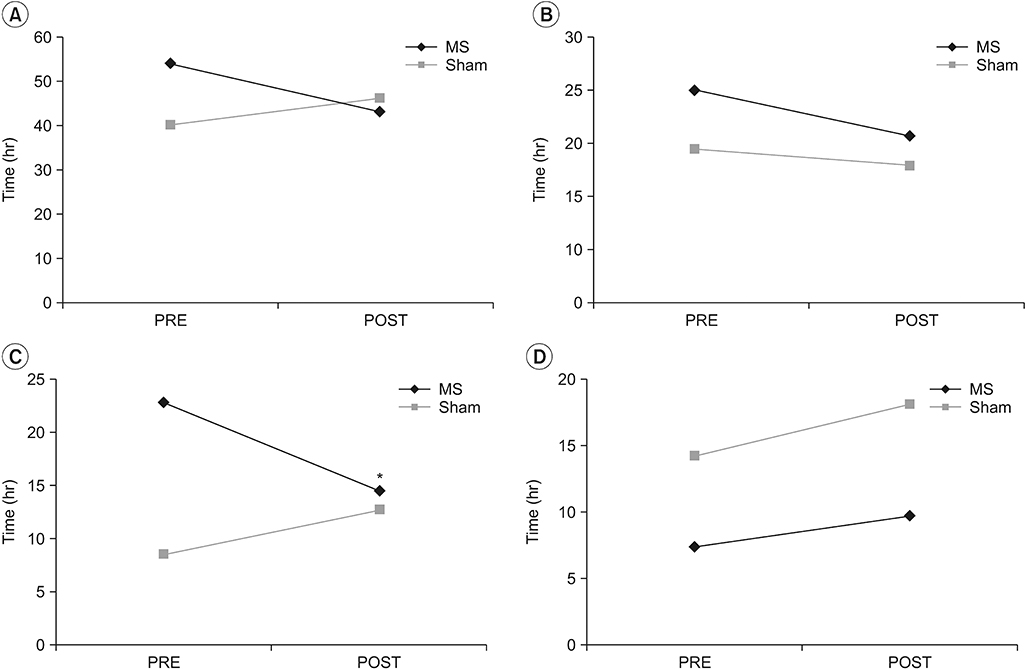
In the MS group, A-FMS was applied to the right colon segment in 6 patients, the left colon segment in 4 patients, and the rectosigmoid colon segment in 2 patients. In the Sham group, A-FMS was applied to the right colon segment in 6 patients, the left colon segment in 1 patient, and the rectosigmoid colon segment in 5 patients. Total CTT decreased from 54±4.9 hours before treatment to 43.2±5.3 hours after treatment in the MS group, and increased from 40.4±6.1 hours before treatment to 46.3±5.7 hours after treatment in the Sham group (Fig. 3A). In the MS group, the segmental CTTs in the right colon and the rectosigmoid colon were 24±4.1 and 7.4±2.5 hours (before treatment) and 18.9±3.6 and 9.9±2.7 hours (after treatment), respectively, and in the Sham group, 17.5±5.6 and 14.4±3.5 hours (before treatment) and 15.4±4.3 and 18.3±4.4 hours (after treatment), respectively (Fig. 3B, 3D). There was no statistical significance between the two groups in total and segmental CTT of the right and rectosigmoid colon. Only the segmental CTT in the left colon was statistically significant between the changes in the MS group (from 22.6±3.8 to 14.4±3.1 hours) and the changes in the Sham group (from 8.5±1.7 to 12.6±2.2 hours) (p = 0.014, Student t-test) (Fig. 3C).
Changes in frequency of defecation (times/week)
The frequency of defecation increased from 1.7±0.7 to 3.2±0.4 weekly in the MS group and from 2.5±0.8 to 3.1±1.0 weekly in the Sham group, which was a significant increase in the MS group compared to the Sham group (p=0.012, Welch test). The number of patients with increased frequency of defecation per week after treatment was 12 in the MS group and 5 in the Sham group. The proportion of patients with increased defecation was significantly higher in the MS group (p<0.01, Fisher exact test).
Changes in the BBS
BBS is classified according to the shapes and hardness of the stool. Types 1 and 2 indicate hard stool whereas Types 3 and 4 indicate normal stool consistency. The types increased from 2.3±0.7 (before treatment) to 3.5±0.5 (after treatment) in the MS group, and from 2.6±0.8 to 3.1±0.5 in the Sham group, respectively. The proportion of patients with an increase in stool types was significantly higher in the MS group compared to the Sham group (10 in the MS group) (p=0.013, chi-square test).
Changes in K-MBI
K-MBI, which conveys that higher the score higher the degree of self-reliance, as a measure of patients’ activities of daily living, was 36.9±31.4 (before treatment) and 41.8±32.0 (after treatment) in the MS group, and 27.8±22.0 (before treatment) and 35.3±30.0 (after treatment) in the Sham group. There was no significant difference in the score between the two groups.
Side effects due to FMS
None of the participants showed any adverse reactions or any other complications during or after treatment with A-FMS.
DISCUSSION
We evaluated the effects of A-FMS on constipation (decrease in large intestine movements) in patients with brain injuries and investigated the effect of CTT, defecation frequency, and stool hardness. Statistically significant changes were observed with regards to several variables. The large intestine is a rather complex organ with autonomic, enteric and somatic nervous input. The vagus nerve (10th cranial nerve, CN X) and pelvic nerve (S2-4) are under the parasympathetic control wherein the vagus nerve innervates the ascending and transverse colons while the pelvic nerve innervates the descending colon. Auerbach’s and Meissner’s plexuses distributed within the enteric system and colonic wall promote peristaltic movement while mixing and advancing the stool in association with the harmonious movement of the bowel. When the stool reaches the rectum, rectal stretching causes simultaneous relaxation of puborectalis muscle and external anal sphincter. Concurrently, the abdominal muscles contract to increase the pressure in the abdomen to promote defecation [12].
FMS was introduced in 1994 to functionally stimulate the peripheral nerves [17]. Changes in the magnetic field induce an electric field and appropriate management of intensity and duration of the electric field generates current sufficient to excite the nerves [18]. Several recent studies have reported improvements in bowel function by stimulating the nerves with magnetic stimulation. Chiu et al. [19] reported a study about the effect of FMS in 16 patients with Parkinson disease during the first 10 minutes for the T9 spinous process for thoracic nerve stimulation and for the remaining 10 minutes for the lumbosacral nerve stimulation in the L3 spinous process for 20 minutes twice daily for 3 weeks with a significant decrease in CTT after application. Wang and Tsai [20] reported improvement in bowel function in 19 patients with intractable constipation aged 65 years or older by magnetic stimulation therapy for 3 weeks by following the protocol described by Chiu et al. [19]. Lin et al. [12] reported that FMS at the aforementioned sites among 13 patients with spinal cord injuries and 2 normal subjects increased rectal pressure and decreased CTT following application of FMS to the abdominal area at 10 cm above the symphysis pubis; they observed a higher rectal pressure compared to the pressure at the time of spinal stimulation and direct smooth muscle contraction as a result of pelvic nerve stimulation. Furthermore, Yoon et al. [13] showed a decrease in CTT, softened stool consistency and increased the frequency of defecation in 12 stroke patients with chronic constipation via transabdominal approach to stimulate pelvic nerve and local enteric system as well as the abdominal muscles directly in a relaxed supine position of these patients. In the present study, we applied the A-FMS through the abdomen while following the protocol described by Yoon et al. [13]. Depending on the frequency of the stimulation, the muscles may repeat the contraction and relaxation in low-frequency setting and maintain a constant contraction state in high-frequency setting. The stimulation frequency of 40 Hz maintains the constant contraction state [21], which leads to physiological defecation activities such as the Valsalva maneuver. A sufficient resting period of 6 seconds after 3 seconds of stimulation was provided to prevent fatigue of the abdominal muscles during 20 minutes of treatment.
The increase in BSS (indicating decreased stool hardness) and the frequency of defecation are the results of complex stimulation of muscles and nerves caused by A-FMS. However, there was no statistically significant difference between the two groups with respect to change in total CTT and the change in segmental CTT except in the segmental CTT in the left colon. Various methods for setting more specific and effective treatment guidelines are necessitated. Herein, in our study we did not evaluate patient’s body mass index or the anteroposterior (AP) diameter of the abdomen—distance from the stimulator to the target structure should be different in each patient—thereby rendering a limitation of the current study.
In conclusion, the present study demonstrated that the use of A-FMS in patients with constipation after brain injury resulted in decreased but equivocal in CTT, increased frequency of defecation and decreased stool hardness. It is proposed that A-FMS should be made available as an additional therapeutic option for the treatment of constipation in brain-injured patients.
Notes
No potential conflict of interest relevant to this article was reported.