Cardiac Rehabilitation Exercise Training for High-Risk Cardiac Patients
Article information
Abstract
Objective
To examine the effect and safety of cardiac rehabilitation (CR) program in high-risk cardiac patients and compare these results to those of control CR participants without high-risk criteria.
Methods
A total of 12 high-risk cardiac patients were recruited as subjects. The high-risk criteria were: advanced heart failure with left ventricular ejection fraction (LVEF) of less than 30%, a recent history of cardiac arrest or dangerous arrhythmia, and cardiac device insertion. Another 12 CR participants without any high-risk criteria mentioned above were recruited as controls. Both groups underwent 6 to 8 weeks of CR exercise training. Exercise tolerance tests were performed before and after completion of the CR program. After CR completion, both groups were evaluated and their results were compared.
Results
After completion of the CR exercise program, both groups showed significant increases in peak oxygen uptake (VO2peak) and LVEF. In the control group (n=12), VO2peak increased from 25.9 to 31.8 mL/kg/min (changing rate, +21.4%±22.1%) and LVEF increased from 56.1% to 59.1% (changing rate, +5.3%±8.4%). In the high-risk group (n=12), VO2peak increased from 16.8 to 21.0 mL/kg/min (changing rate, +28.6%±21.4%) and LVEF increased from 26.1% to 29.4% (changing rate, +16.1%±12.9%). There was no serious cardiovascular event during all exercise hours.
Conclusion
High-risk cardiac patients who completed a supervised CR program demonstrated significant improvements in VO2peak and LVEF without any serious cardiovascular event. The improvement rate was similar to that of control group.
INTRODUCTION
There is a growing consensus that exercise has a beneficial effect on patients with cardiovascular disease, even for those with severely impaired cardiac function because physical inactivity accelerates the severity of heart failure (HF) [1]. Patients with chronic heart failure (CHF) have a reduced exercise tolerance as a result of several abnormalities in multiple organ systems. The reduced exercise tolerance further deteriorates exercise capacity, creating a vicious cycle of progressive deconditioning and worsening of HF [2]. In the last decade, several studies have demonstrated that HF patients in the New York Heart Association functional class II and III can benefit from moderate exercise training with significant improvements in exercise capacity, quality of life, and reduction in hospitalizations [34].
Implantable cardioverter defibrillator (ICD) can improve the survival of patients with HF and significant left ventricular dysfunction [56]. Patients with ICDs frequently have fear over receiving shock during exercise. The fear of inappropriate shocks is a commonly cited cause when ICD patients are denied referral to an exercise training program [7]. However, due to the benefit of exercise, the ACC/AHA HF Guidelines has recommended exercise training [8]. More recently, the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial, a multicenter randomized controlled trial enrolling 2,331 medically stable outpatients with CHF, has demonstrated a smaller but significant improvement in peak oxygen uptake (VO2peak) at three months which has persisted to 12 months [9]. Many pioneers have applied exercise training in high-risk patients and documented the safety and beneficial effects of exercise training on those patients [91011].
The aim of this study was to determine the effect and safety of cardiac rehabilitation (CR) program in high-risk patients, including advanced HF, history of life-threatening arrhythmias, and implanted ICD and/or cardiac resynchronization therapy (CRT). We compared and analyzed the results of CR program between high-risk and non-high-risk cardiac patients through reviewing medical records retrospectively.
MATERIALS AND METHODS
Subjects
We enrolled patients who visited a Cardiac Rehabilitation Clinic between January 2012 and December 2015. Their medical records were reviewed and analyzed retrospectively. Following the risk stratification published by the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) guidelines [12], CR participants with a high-risk for cardiovascular events during exercise training were included as study subjects. High-risk patients were defined as those with advanced HF (left ventricular ejection fraction [LVEF] <30%), or a recent history of cardiac arrest or dangerous arrhythmia such as sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), and early periods of cardiac device insertion including ICD or ICD with CRT. Patients who received PCI for an acute MI without any high-risk criteria mentioned above (n=121) were included as control group. In order to minimize the selection bias, matched control groups were selected based on gender, age, and basic demographic data during the same period (n=64). These control groups were randomized by an Excel program to match the number (n=12) of the high-risk group. Twelve patients were enrolled for the high-risk group. The control group also included 12 patients. They all completed the exercise program. Pre-exercise reviews of clinical data including the results of a transthoracic echocardiogram (TTE) and/or a pacemaker summary were performed. Medications did not change during the 6 to 8 weeks study period. This study was performed according to the Declaration of Helsinki. It was approved by the Sanggye Paik Hospital Institutional Review Board (IRB No. 2016-02-008).
Exercise testing
All study subjects received an exercise tolerance test (ETT) and a baseline test. Follow-up tests were performed after completing 6 to 8 weeks of exercise training. An ETT was conducted to evaluate the cardiovascular response to exercise to ensure that the patient could tolerate the exercise in both groups. A symptom-limited ETT was conducted using a modified Bruce protocol. A real-time recording 12-channel electrocardiograph (ECG) (Q4500; Quinton Instrument Co., Boston, MA, USA); a respiratory gas analyzer (TrueOne 2400 Metabolic Measurement System; ParvoMedics, Inc., East Sandy, UT, USA); an automatic blood pressure (BP) and pulse monitor (Model 412, Quinton Instrument) and a treadmill (Medtrack ST 55, Quinton Instrument) were used for the ETT. The ETT measured a VO2peak, duration of exercise and submaximal myocardial oxygen demand (MVO2). VO2peak was measured with a respiratory gas analyzer. Peak heart rate, resting heart rate, and MVO2 were estimated with the ECG and automatic BP and pulse monitor. The MVO2 was calculated by multiplying systolic BP and heart rate as a rate pressure product (RPP). Submaximal MVO2 was measured at the end of stage III of the modified Bruce protocol.
Exercise training
Both groups participated in an ambulatory supervised exercise training program for 6 to 8 weeks. One experienced physical therapist who specialized in CR supervised the training sessions. Three exercise sessions per week were offered. The duration of each session was approximately 60 minutes. Exercise intensity of 60% to 85% heart rate reserve (HRR) was individually calculated for each patient using the Karvonen formula ([maximal heart rate–resting heart rate×%intensity]cresting heart rate). The target heart rate was calculated at 60% of the HRR during the first 2 weeks, at 70% of HRR during the third and fourth weeks, and at 85% of HRR during the remained periods. ICD patients were instructed not to surpass an upper heart rate threshold. This threshold was set at the detection rate of ICD minus 30 beats/min. During the training sessions, individual BP, ECG, and heart rate were strictly monitored. The exercise stop criteria included in the American Heart Association guidelines were strictly followed during exercises [12].
Statistical analysis
Data analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Data is reported as a mean±standard deviation unless otherwise stated. When comparing two groups, paired t-tests or Mann-Whitney U-tests were used depending on the normality of distribution. To compare gender, smoking status, and drug history, Pearson chi-square tests and Fisher exact tests were used. To compare the effects of exercise between the two groups, a Wilcoxon signed-rank test was used. A repeated two-way analysis of variance (ANOVA) was used to test between-group differences in the changes of outcome variables from before to after exercise training. All tests were two-tailed and a p-value of less than 0.05 was considered as statistically significant.
RESULTS
Patient characteristics
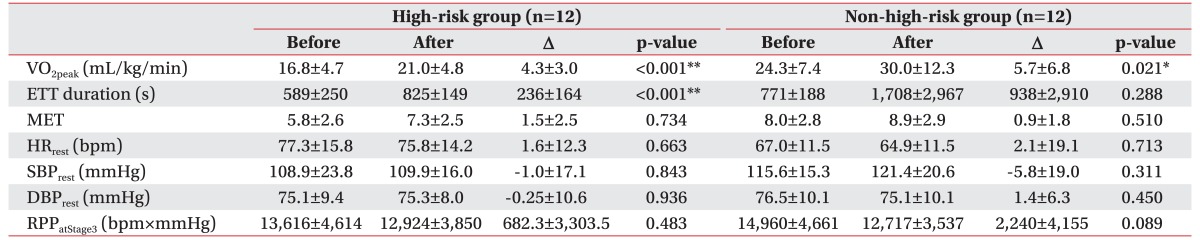
Characteristics of the 12 patients with the high-risk group and the 12 control patients are summarized in Table 1. The high-risk group consisted of 9 males and 3 females, while the control group consisted of 10 males and 2 females. There was no significant difference in the distribution of gender, age, diabetes, or smoking status between the two groups. However, drugs, beta blockers, statins, and aspirin were generally taken more by the control group. This is due to the fact that most patients in the control group have acute coronary syndrome. According to the baseline laboratory parameters, LVEF and VO2peak in the high-risk group were significantly lower than those in the control group.
The high-risk group included 11 advanced HF patients (<20% in 6 cases) and one sudden cardiac death syndrome. Five of them received ICD including one CRT (Table 2). The LVEF of HF patients was all under 30 percent. The most common reason for inserting an ICD or ICD with CRT was ventricular fibrillation.
Data comparison before and after the cardiac rehabilitation program
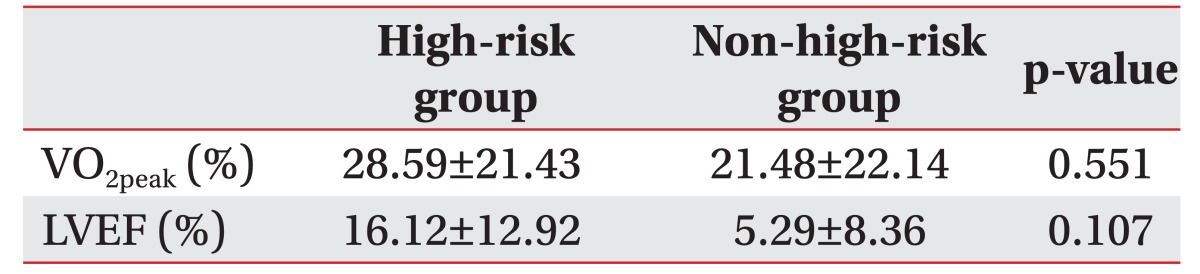
All patients completed the six to eight weeks of cardiac rehabilitation training program. After exercise training, the VO2peak was increased from an average of 16.8 to 21.0 mL/kg/min in the high-risk group and from 24.3 to 30.0 mL/kg/min in the non-high-risk group (Table 3). Such an increase was statistically significant (p<0.05) in both groups. The rate of change in VO2peak was 28.6% in the high-risk group, which was higher than 21.5% in the non-high-risk group (Table 4). However, such a difference was not statistically significant (p>0.05). Exercise tolerance duration was significantly increased in the high-risk group. Individual values of VO2peak before and after the training in each group are shown in Fig. 1.
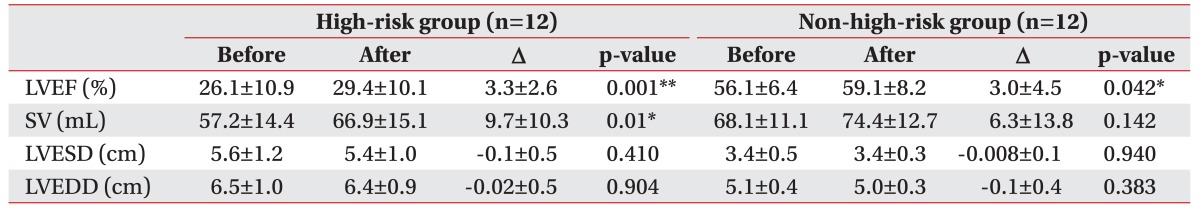
After 6 to 8 weeks of CR exercise training, the LVEF was increased from the average of 26.1% to 29.4% in the high-risk group and from 56.1% to 59.1% in the non-high-risk group (Table 5). The increase was statistically significant (p<0.05) in both groups. The rate of change in LVEF was 16.1% in the high-risk group, which was higher than 5.3% in the non-high-risk group (Table 4). However, such a difference was not statistically significant (p>0.05). Stroke volume significantly (p<0.05) increased in the high-risk group. The individual values of LVEF before and after the training in each group are shown in Fig. 2.
Cardiovascular-related complications during exercise monitoring
During the intervention period, there was no adverse cardiovascular event and symptomatic arrhythmias. Fatal cardiac complications such as cardiac arrest, death, and myocardial infarction were not observed. No inappropriate discharge was delivered by the ICDs at any time during exercise testing or training. No patient experienced any complication that required immediate medical attention during the exercise testing or training. All the 24 subjects finished the CR program.
DISCUSSION
The AACVPR guideline 2004 published risk stratification for cardiovascular complications that can develop with exercise [13]. According to this stratification, the high-risk group was defined as patients with advanced HF (LVEF<30%), a recent history of cardiac arrest or dangerous arrhythmia such as sustained VT or VF, and early periods of cardiac device insertion including ICD or ICD with CRT.
In the present study, exercise capacities before and after training were compared between high-risk group of patients (n=12) and a control group of cardiac patients (n=12). The exercise program was feasible. It was performed by the 12 high-risk patients for 6 to 8 weeks without any arrhythmic event. In previous studies, exercise testing of patients with a history of malignant arrhythmias has shown high rates of arrhythmic complications [14]. It has been reported that the risk of cardiovascular complications during exercise testing and exercise training is higher in patients with a history of threatening arrhythmias or cardiac arrest [1415]. A few studies have reported the safety and feasibility of physical activity and exercise-related complications in patients with malignant ventricular arrhythmias [1416]. In our study, no serious cardiovascular complication such as cardiac arrest and any arrhythmia with hemodynamic compromise was observed, suggesting that careful monitoring during exercise training and prescription of a suitable exercise program can effectively decrease the risk and fear of exercise-related complications. Therefore, CR could be actively applied to high-risk patients.
Several cases have also described inappropriate ICD discharges in patients during exercise [1718]. The fear of inappropriate shocks is a commonly cited reason when ICD patients are denied referral to an exercise training program [7]. A recent study has revealed that the risk for ICD shock related to a traditional exercise training program is low as most of these patients have been revascularized after undergoing stress testing prior to exercise training [19]. Piccini et al. [20] have performed a controlled trial of exercise training. The trial included a total of 2,331 randomized patients with HF and an ejection fraction of ≤35% to exercise training or usual care. A total of 108 (20%) of the exercise patients had shock while 113 (22%) of the control patients had shock. There was no evidence of increased ICD shocks or reduced left ventricular function for those who underwent exercise training, indicating that exercise therapy for ICD recipients with HF should not be prohibited.
In our study, no inappropriate discharge was delivered by the ICDs at any time during exercise testing or training. Furthermore, no patient experienced any complication that required immediate medical attention during exercise testing or during training. The initial device adjustments to ensure a safety threshold (from inducible HR to ICD shock threshold) might have reduced the risk of inappropriate shocks during exercise.
Aerobic exercise capacity measured as VO2peak has been shown to be the strongest predictor of both all-cause mortality and cardiac mortality among patients with cardiovascular disease [121]. This study revealed that the values of VO2peak and LVEF were significantly increased in both groups after cardiac rehabilitation, suggesting that exercise capacity could be increased after exercise training and a CR program, even in high-risk patients. Particularly, the high increase of VO2peak, which is closely related to survival rates might bring great benefit to high-risk patients. The change rate of VO2peak was 28.6% in the high-risk group which was much higher than that 21.4% in the non-high-risk group, although the difference was not statistically significant. The higher change rate of VO2peak in the high-risk group might be due to the lower initial VO2peak in the high-risk group compared to that in the non high-risk group. This result is consistent with results of previous studies showing a higher increase in the rate of in VO2peak after a cardiac rehabilitation program with a lower initial VO2peak [22].
It has been shown that regular exercise training can lead to a modest but significant decline in cardiac size and an improvement in left ventricular function in patients with moderate CHF [23]. However, our study revealed that exercise training was more effective in patients with advanced HF compared to control cardiac patients. The LVEF was improved. However, there was no significant change in left ventricular end-diastolic volume (LVEDV). These results suggested that LVEF might have been improved by the enhancement of intestinal/splenic venoconstriction, in agreement with the result of a previous study showing improved autonomic function after exercise training [24]. It has been previously shown that exercise training can lead to partial correction of peripheral endothelial dysfunction in patients with moderate CHF [25]. Given that the vascular tone of peripheral arteries is one component of afterload, it is obvious that the improvement in endothelial function observed after aerobic endurance exercise training is inversely correlated with the decline in systemic vascular resistance as shown in a previous study [23]. Therefore, the improvement in left ventricular function is at least partially the result of an exercise training–induced decline in afterload.
This study had the following limitations. First, our study was conducted retrospectively using medical records of subject patients. For this reason, a selection bias cannot be ruled out. Second, the number of subjects was quite small which limited the study's validity on safety. Third, this study did not have a longer follow-up after 8 weeks. Therefore, the long-term improvement in exercise capacity after the exercise program could not be confirmed. Despite these limitations, this study safely implemented CR in patients with high-risk for cardiac events and found obvious improvement in exercise capacity.
In conclusion, high-risk cardiac patients who completed a 6- to 8-week supervised CR program demonstrated significant improvement in VO2peak and LVEF without any serious cardiovascular event or negative remodeling of left ventricular wall. There was no statistically significant difference in the rate of improvement between the high-risk cardiac group and the control group without high-risk. The present study suggests that exercise training in high-risk patients is feasible and safe. It can provide a cumulative benefit in terms of exercise capacity. Therefore, exercise training for high-risk patients can be encouraged in clinical applications. More study is needed to further elaborate the role of CR in this patient cohort with a larger number of patients.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.