- Search
| Ann Rehabil Med > Volume 41(4); 2017 > Article |
Abstract
Objective
To report the characteristics of myofascial trigger points (MTrPs) in the infraspinatus muscle and evaluate the therapeutic effect of trigger-point injections.
Methods
Medical records of 297 patients (221 women; age, 53.9±11.3 years) with MTrPs in the infraspinatus muscle were reviewed retrospectively. Because there were 83 patients with MTrPs in both infraspinatus muscles, the characteristics of total 380 infraspinatus muscles with MTrPs (214 one side, 83 both sides) were investigated. Specific characteristics collected included chief complaint area, referred pain pattern, the number of local twitch responses, and distribution of MTrPs in the muscle. For statistical analysis, the paired t-test was used to compare a visual analogue scale (VAS) before and 2 weeks after the first injection.
Results
The most common chief complaint area of MTrPs in the infraspinatus muscle was the scapular area. The most common pattern of referred pain was the anterolateral aspect of the arm (above the elbow). Active MTrPs were multiple rather than single in the infraspinatus muscle. MTrPs were frequently in the center of the muscle. Trigger-point injection of the infraspinatus muscle significantly decreased the pain intensity. Mean VAS score decreased significantly after the first injection compared to the baseline (7.11 vs. 3.74; p<0.001).
Nonspecific complaints of pain near the scapula are commonly encountered, and may originate from any disorder of the cervical spine or shoulder joint. In some cases, however, pain near the scapula appears without any relation to the cervical spine or shoulder, and additional clinical studies are required to reveal possible causes of pain and to find the effective treatments.
Myofascial trigger points (MTrPs) may offer an alternative explanation for the pathophysiological mechanism of scapular pain. Although MTrPs are rarely a primary origin of pain [1,2], evidence suggests that MTrPs are caused by or related to a lesion in another soft tissue, such as spine disorder or rotator cuff disease [2,3]. MTrPs are local points that are highly sensitive to pressure. Their palpation causes characteristic referred sensations, including pain, muscle dysfunction, and sympathetic hyperactivity [4,5,6]. MTrPs are classified into active and latent. Active MTrPs present clinical pain with or without activity associated with tenderness in a taut band, whereas latent MTrPs are clinically quiescent and are painful only when palpated.
Based on our clinical experiences, many patients with or without spine or rotator cuff diseases also have scapular pains that originate in the infraspinatus muscle. The infraspinatus muscle, a thick and triangular muscle, occupies the chief part of the infraspinatus fossa and assists external rotation of the arm and stabilization of the humerus head during an arm movement [7]. Active MTrPs in this muscle cause both local pain and referred pain in the shoulder region and down to the frontal and lateral side of the arm [5].
The infraspinatus muscle frequently harbors MTrPs. In one study with 126 patients, referred pain to the shoulder region arose from the infraspinatus muscle in 31% of the cases, a frequency second only to that of the levator scapulae (55%) [8]. Another study reported similar results [9]. Among young, pain-free adults, the infraspinatus muscle was the third (18%) in the prevalence of latent MTrPs, following the levator scapulae (20%) and the upper trapezius (35%) [10].
We recently observed many patients who were compatible with the criteria of MTrPs in the infraspinatus muscle, as assessed by careful history taking and physical examination. In treating these patients, we found some differences from previous studies [11] in the chief complaint area, referred pain pattern, distribution of MTrPs, and therapeutic effect of trigger-point injection.
The objectives of this study were to report on the characteristics of MTrPs in the infraspinatus muscle.
After Institutional Review Board approval, we retrospectively reviewed the medical records of 446 patients with suspected MTrPs in the infraspinatus muscle who had visited the outpatient clinic of Severance Hospital between March 2013 and November 2015 in order to identify all eligible patients (4-2015-1049).
Diagnosis of active MTrPs was based on prior criteria, including tender spots in the infraspinatus muscle, a compatible pattern of referred pain elicited when tender spots were compressed, restricted range of motion of the shoulder, and palpable or visible local twitch responses (LTRs) at the most sensitive spot in the taut band. LTRs were elicited and assessed during trigger-point injections. Since needling is more sensitive than manual palpation in identifying LTRs, the needling method was chosen to confirm the presence of MTrPs.
Among patients with MTrPs in the infraspinatus muscle, those who met the following criteria were included in this study: MTrPs of muscle and fascia, exclusive of enthesopathy; pain score measured by a visual analog scale (VAS) greater than 2 (≥3) on a numeric scale of 0−10; and having a trigger-point injection. Patients were excluded if they had: (1) received treatment that could affect the result (such as pain killers, physical therapy, or other injection therapy) from 2 weeks before intervention and through the follow-up period; (2) some other systemic disease that could present the same symptom (e.g., fibromyalgia); (3) neurologic shoulder/scapular pain (e.g., cervical radiculopathy or peripheral nerve injury); and (4) previous history of an adverse effect of lidocaine or steroid, or any conditions or situations that might place the patient at significant risk during the injection.
However, if the patient met the criteria of MTrPs in the infraspinatus muscle, they were not excluded even if they had a history of the rotator cuff and/or cervical spine disease.
All injections were performed under ultrasound (US)-guided injection. However, this retrospective design of the study was not to evaluate the usefulness of US-guided injection of the infraspinatus. The US-guided trigger-point injection method previously reported for the lower back muscles, deep pelvic muscles, and the brachialis muscles was modified for MTrPs in the infraspinatus muscles [12,13,14]. We performed B-mode, real-time US in a sterile environment using an Accuvix V10 US machine(Medison, Seoul, Korea) interfaced with a 5- to 12-MHz linear array transducer around the target muscle. A physiatrist with more than 8 years of experience in the musculoskeletal US carried out all the US-guided injection procedures.
The patients lay prone on an examination bed with the affected arm extended, internally rotated, and adducted, and try to reach the thoracic spine—that is, in a position similar to the surgical “chicken wing” position for injection procedures (Fig. 1). With this position, we could easily and safely approach the infraspinatus muscle by separating the scapular bone from the surrounding tissues.
We differentiated the infraspinatus muscle from the deltoid, trapezius, teres major, teres minor, and latissimus dorsi muscles by US scanning and marked the tender points on the skin by palpating the muscle. By positioning the probe on the marked points and turning the probe for the best view, we obtained the image of the target area (Figs. 1, 2). Color Doppler images were used to avoid the neurovascular bundle. Under US guidance, a 25-gauge, 2.6-cm needle connected to a 5-mL syringe containing a mixture of 4 mL of 0.5% lidocaine and 1 mL of 40 mg of triamcinolone was inserted into the infraspinatus muscle at the region where the tender spot was palpated. In the case of multiple tender spots in one muscle, the injections were repeated for all tender spots. The sequence of the injection sites was determined at each treatment period in terms of the decreasing order of pain severity in the tender points when palpated. The injection volume per tender point was 0.5 mL. No more injections were done once all the prepared solution of 5 mL had been used up (at most, there could be 10 injections per treatment session) or when no more palpated tender point were found. With the use of an out-of-plane method, we could see the needle passing through the skin and adipose tissue to penetrate the muscle. The physiatrist observed the LTRs on the US while performing the trigger-point injections. The needling was repeated to elicit as many LTRs as possible. If no LTR was observed after 10 attempts, the needling was stopped and a mixture solution was injected. The injection site was pressed to ensure proper hemostasis after the procedure. Trigger-point injections were carried out in all MTrPs of the muscle at 2-week intervals. Additional injections were not considered if patients were satisfied with the reduction in discomfort or pain severity, or if patients did not want another injection for some reason. The patients were taught to do stretching exercise (repeated 10−20 times daily) and to avoid any posture that might aggravate the symptoms.
Demographic and clinical data including age, gender, affected side, and the duration of symptoms were collected. Pain intensity, duration of symptoms, chief complaint area, referred pain pattern, the number of injections, and the number of LTRs were recorded, and the distribution of MTrPs in the muscle was established in terms of the sites of LTRs that were given US-guided injections (Fig. 3). Pain intensity was measured by using a 10-cm horizontal VAS, which ranged from 0 (no pain) to 10 (worst imaginable pain). VAS was assessed at baseline and at 2 weeks after each injection.
We carried out descriptive analyses of patient information and characteristics of MTrPs in the infraspinatus muscle. For patients with MTrPs in both infraspinatus muscles, pain intensity, duration of symptoms, chief complaint area, referred pain pattern, the number of injections, and the number of LTRs were evaluated on each side. Evaluation of the effect of the trigger-point injections used the Shapiro-Wilk test of the VAS data acquired at baseline and at 2 weeks after the first injection to find out whether the distribution was normal. When the Shapiro-Wilk test showed a normal distribution for these parameters, a paired t-test was used to compare parameters before and 2 weeks after the first injection. Statistical significance was set at a p-value of <0.05. SPSS ver. 21.0 software (IBM, Armonk, NY, USA) was used for all statistical analyses.
We reviewed the medical records of 446 patients with suspected MTrPs in the infraspinatus muscles between March 2013 and November 2015. Of these, 297 patients (221 females and 76 males) were eligible for this study. Because there were 83 patients with MTrPs in both infraspinatus muscles, there were 380 cases of infraspinatus muscles with MTrPs that satisfied the inclusion criteria. In 83 patients with MTrPs in both infraspinatus muscles, both muscles were evaluated separately for the duration, pain severity, chief complaint area, and pattern of referred pain. The demographic and clinical characteristics of 297 patients and the clinical characteristics of the 380 infraspinatus muscles with MTrPs are summarized in Table 1.
Among the 297 patients, there were 103 with a medical history of shoulder disease, including rotator cuff disease (n=103, 34.7%), sports or work-related injury without shoulder and cervical spine disease (n=89, 30.3%). cervical spine disease (n=61, 20.5%), and shoulder disease combined with cervical spine disease (n=44, 14.8%). Among the patients with work-related injury, we found that 17 (5.7%) took care of the children.
In the 380 infraspinatus muscles with MTrPs, the most common chief complaint area was the scapular area; the next most common was deep in the front of the shoulder (Table 1). One hundred thirty-one cases had referred pain except in the areas of chief complaint ; the most common area was the anterolateral aspect of the arm (above the elbow) (Table 2).
The LTRs necessary for diagnosing MTrPs were identified in all 380 infraspinatus muscles. However, the number of LTRs was recorded in the medical charts for only 153 cases. Of these, 38 cases (24.8%) had fewer than 5 LTRs. Cases with 6–10, 11–15, 16–20, and >10 LTRs were 45 (29.4%), 46 (30.1%), 24 (15.7%), and 70 (45.8%), respectively.
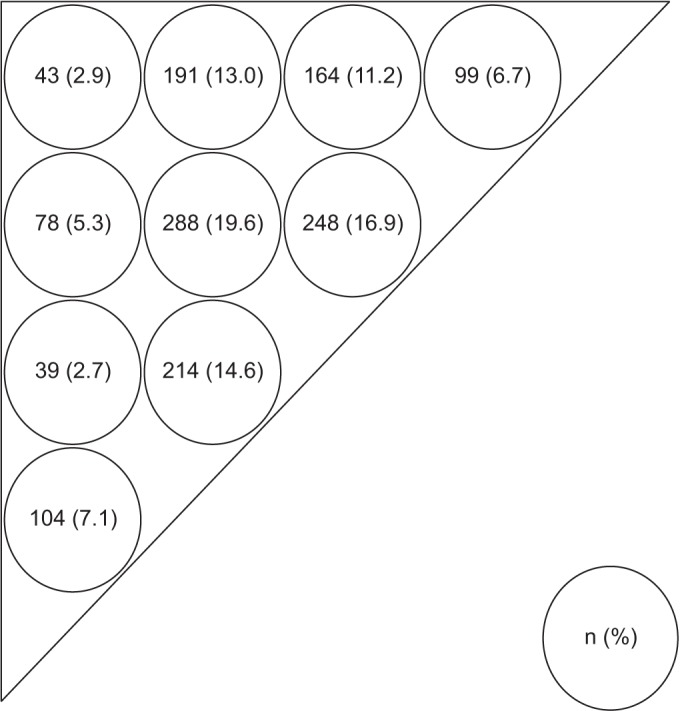
Fig. 3 summarizes the distribution of MTrPs in the infraspinatus muscles based on the scapular bone in 297 patients. For all 380 cases, 1,468 MTrPs were found, averaging 4.9 MTrPs per muscle.
Among the 380 cases, the effect of the trigger-point injections was assessed for 135 cases. One injection was given to 16 (11.9%) of the 135 patients, 2 injections at an interval of 2 weeks for 88 patients (65.2%), and 3 injections every 2 weeks for 31 patients (23.0%). There was a significant decrease in the VAS at 2 weeks after the first injection compared to the baseline (p<0.001). The mean value decreased significantly from 7.11 (SD=1.45) pretreatment to 3.74 (SD=1.53) after the first treatment.
There are three important findings in this study. First, one of the main symptoms of MTrPs in the infraspinatus muscle is scapular pain. Second, active MTrPs were almost always multiple rather than single in the infraspinatus muscle on the painful side. Third, inactivation of active MTrPs in the infraspinatus muscle significantly decreased pain intensity.
The main symptom of MTrPs in the infraspinatus muscle was pain deep in the front of the shoulder, including the anterior deltoid area, in previous studies [9,15,16,17,18]. Although the pain from MTrPs was frequent deep in the front of the shoulder, pain in the scapular area was more common in this study. These findings may depend on the differences in the definition of the MTrPs, the patient population, and the exactitude of the questions asked to patients during the medical examination.
First, MTrPs can exist within skeletal muscle tissue, aponeurosis (fascia) of the muscle, or the tendon, but this study was restricted to MTrPs within the infraspinatus muscle tissue and/or fascia of the muscle, which is the most common type of MTrPs [19]. Second, we did not exclude patients who had a history of cervical spine disease or shoulder lesion if the patients met the criteria of MTrPs in the infraspinatus muscle. Third, because patients may not be able to distinguish scapular pain from shoulder pain, the physiatrist asked questions to find out if the pain presented exactly around the scapular area during the medical examination. If the physiatrist did not ask specifically about scapular pain, a patient could have thought the question was about shoulder pain.
Referred pain is a pain felt in other than the true site of origin [20]. In this study, the locations of referred pain from MTrPs in the infraspinatus muscle were, in order of frequency, the anterolateral aspect of the arm (above the elbow), the lateral forearm, the upper posterior neck, and the radial aspect of the hand (including a finger). Our results were similar to those of a study of 193 patients, in which the anterolateral aspect of the arm (46%) was the most frequent site of the referred pain, followed by the lateral forearm (21%), the posterior neck (14%), and the radial aspect of the hand (13%) [9,11,15,16,18,21,22]. When the referred pain is on the radial aspect of the hand, it was especially important to differentiate it from cervical radiculopathy, mainly at the C5 or C6 level [23]. The range and site of the referred pain can depend on the location and intensity of the MTrPs in the infraspinatus muscle, and further study is required. MTrPs in the infraspinatus and teres minor muscles often occur together and are often incorrectly assessed as rotator cuff lesions or cervical discogenic pain. In addition, MTrPs in the infraspinatus muscle are often incorrectly assessed as osteoarthritis of the shoulder joint, entrapment of the suprascapular nerve, or bicipital tendinitis [19]. Also, MTrPs in the infraspinatus muscle should be differentiated from those in the teres major, supraspinatus, anterior deltoid, subscapularis, and pectoralis major muscles [19].
One of the main contributions of our study is the finding that there were multiple, not just single, active MTrPs in the infraspinatus muscle on the painful side. Furthermore, there are many LTRs per infraspinatus muscle, and cases of more than 10 LTRs were frequently observed (45.8%). If LTRs are elicited during injection, especially if the fast-in–fast-out technique was used, immediate pain relief could be achieved more often [24,25]. Patients can fail to experience immediate and complete pain relief if the LTRs are not elicited during a trigger-point injection. After injection into one responsive locus, other LTRs can be elicited. These procedures should be repeated until all (or as many as possible) responsive loci are injected [26]. To our knowledge, the number of LTRs has not been reported previously. Therefore, these results highlight the importance of searching for multiple active MTrPs regions and LTRs within one muscle in patients with myofascial pain syndrome in the infraspinatus muscle. Multiple MTrPs in the same muscle can each contribute to the overall referred pain pattern.
Trigger-point injection of the infraspinatus muscle resulted in excellent outcomes, and VAS scores decreased after treatment. We suggest that, in a patient with MTrPs in the infraspinatus muscle, trigger-point injection of the infraspinatus is effective for both diagnosis and treatment when the scapular pain is suspected to originate from the infraspinatus muscle.
MTrPs in the infraspinatus are usually activated by an acute stress or by multiple overload stresses. These MTrPs may become active and induce pain under the influence of certain perpetuating factors, such as repetitive and sustained shoulder activities [27,28]. This could be the explanation for the 17 patients without shoulder lesion who took care of children. When the shoulder is abducted and flexed, the infraspinatus muscle shows less activity than the supraspinatus muscle [29]. However, there is a marked increase of infraspinatus activity at over 140° of abduction. Therefore, when the patient carries heavy loads for a long time with the shoulder abducted above the acromion level, the infraspinatus muscle could be damaged. These mechanisms could explain the cases of MPS in women without underlying disease who were actively involved in child care. Since the infraspinatus muscle, unlike the supraspinatus muscle, is likely to be strongly activated in movements that are unusual and transient, acute overload could be much more likely to develop MTrPs than tasks that impose a sustained overload [11].
The muscle imbalance may also keep the MTrPs active and induce recurrent pain. Since MTrPs can induce changes in normal muscle-activation patterns and subsequent motor dysfunction, identifying and inactivating MTrPs should improve motor function, release muscle stiffness, and restore normal biomechanics of the shoulder [30].
It is important to know the common sites of MTrPs in the infraspinatus muscle in order to provide guidance on the clinical identification of MTrPs in this muscle. Careful palpation frequently discloses multiple tender spots in the infraspinatus muscle as indicated by the multiple lesions. In previous studies, the most common MTrPs region was caudal to the junction of the most medial and adjacent quarter of the length of the scapular spine (upper medial lesion) [11]. The next most common MTrPs region was caudal to the midpoint of the scapular spine (lateral upper lesion) [11]. In this study, the common MTrPs region was similar to that found by Travell, but was more broadly positioned, and the inferior angle of the scapula was more frequently observed. Because the infraspinatus muscle is quite broad and palpating all of it during the physical examination could be time-consuming, this study could provide a helpful guideline for identifying MTrPs in the infraspinatus muscle.
US-guided injection is useful for detecting LTRs in deeply located muscles and for controlling the depth during injection for even less-accessible muscles. US guidance can also reduce inadvertent injuries that could be caused by improper needle placement. It might seem unnecessary to use US-guided injection, because the infraspinatus muscle is located superficially, and the needle is less likely to injure surrounding tissues, such as the lung, inadvertently. However, by using US-guided injection, we could observe more LTRs to improve the effects of injection, differentiate the neighboring teres major, deltoid, and trapezius muscles in order to diagnose more accurately, and provide feedback for the treatment by recording with built-in video. These strengths could be worthy of attention.
There are some limitations to be considered in our study. First, because the study was retrospective, we could have missed information required for accurate analysis.
Second, we conclude the therapeutic effects of trigger-point injections without comparison to a control group. Nevertheless, we could not ignore the significant decrease in the VAS scale (more than 3 points) from injections during short-term treatment.
Third, we investigated only one referred pain pattern per case. If multiple tender points exist, there could be many different referred pain patterns depending on the location, number, and pain severity of the tender points. This is also a limitation of a retrospective study, which could be complemented by further prospective studies.
Fourth, the follow-up period of the therapeutic effect of the trigger-point injections was relatively short. Although there was no long-term follow-up, the long-term effects of trigger-point injections for MPS in the infraspinatus muscle would not differ from those for other muscles. If the underlying etiologic lesion cannot be eliminated, the effect of one trigger-point injection usually lasts about 2 weeks [13]. Inactivation of active MTrPs, however, is necessary for some situations, including the presence of severe and intolerable pain, pain or discomfort that interferes with functional activities, and persistent pain and tightness. The same principle can be applied to the infraspinatus muscle.
Fifth, the selected group of patients was not homogeneous. In particular, some patients with shoulder or cervical spine lesions were included in this study. MTrPs could be secondary to pathologic conditions such as chronic repetitive minor muscle strain, poor posture, systemic diseases, and musculoskeletal lesions (such as strain, sprain, enthesopathy, bursitis, arthritis, and spinal disc lesion) [2,3]. But for the patients with a history of shoulder or cervical spine lesion in our study, their lesions were not their main reason for visiting a clinic. In addition, their symptoms satisfied MTrPs criteria and, ironically, their MTrPs were less frequent than for patients without a history of shoulder or cervical spine lesion [1].
In conclusion, our findings of MTrPs in the infraspinatus muscle and the therapeutic effect of trigger-point injections in that muscle may provide clinicians with useful information in diagnosing and treating myofascial pain syndrome of the infraspinatus muscle.
CONFLICT OF INTEREST:
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
1. Hong CZ. Pathophysiology of myofascial trigger point. J Formos Med Assoc 1996;95:93-104. PMID: 9064014.

2. Hong CZ, Simons DG. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil 1998;79:863-872. PMID: 9685106.


4. Zhang Y, Ge HY, Yue SW, Kimura Y, Arendt-Nielsen L. Attenuated skin blood flow response to nociceptive stimulation of latent myofascial trigger points. Arch Phys Med Rehabil 2009;90:325-332. PMID: 19236988.


5. Ge HY, Fernandez-de-las-Penas C, Arendt-Nielsen L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin Neurophysiol 2006;117:1545-1550. PMID: 16737848.


6. Kimura Y, Ge HY, Zhang Y, Kimura M, Sumikura H, Arendt-Nielsen L. Evaluation of sympathetic vasoconstrictor response following nociceptive stimulation of latent myofascial trigger points in humans. Acta Physiol (Oxf) 2009;196:411-417. PMID: 19210492.


7. Mochizuki T, Sugaya H, Uomizu M, Maeda K, Matsuki K, Sekiya I, et al. Humeral insertion of the supraspinatus and infraspinatus: new anatomical findings regarding the footprint of the rotator cuff: surgical technique. J Bone Joint Surg Am 2009;91(Suppl 2 Pt 1): 1-7.

8. Sola AE, Kuitert JH. Myofascial trigger point pain in the neck and shoulder girdle: report of 100 cases treated by injection of normal saline. Northwest Med 1955;54:980-984. PMID: 13253967.

9. Pace JB. Commonly overlooked pain syndromes responsive to simple therapy. Postgrad Med 1975;58:107-113.

10. Sola AE, Rodenberger ML, Gettys BB. Incidence of hypersensitive areas in posterior shoulder muscles: a survey of two hundred young adults. Am J Phys Med 1955;34:585-590. PMID: 13268620.

11. Travell J, Simons D, Simons L. Myofascial pain and dysfunction: the trigger point manual (Vol. 2: The lower extremities). Baltimore: Williams & Wilkins; 1999.
12. Rha DW, Shin JC, Kim YK, Jung JH, Kim YU, Lee SC. Detecting local twitch responses of myofascial trigger points in the lower-back muscles using ultrasonography. Arch Phys Med Rehabil 2011;92:1576-1580. PMID: 21839982.


13. Kim DS, Jeong TY, Kim YK, Chang WH, Yoon JG, Lee SC. Usefulness of a myofascial trigger point injection for groin pain in patients with chronic prostatitis/chronic pelvic pain syndrome: a pilot study. Arch Phys Med Rehabil 2013;94:930-936. PMID: 23262156.


14. Suh MR, Chang WH, Choi HS, Lee SC. Ultrasound-guided myofascial trigger point injection into brachialis muscle for rotator cuff disease patients with upper arm pain: a pilot study. Ann Rehabil Med 2014;38:673-681. PMID: 25379497.



15. Rubin D. Myofascial trigger point syndromes: an approach to management. Arch Phys Med Rehabil 1981;62:107-110. PMID: 6453568.

17. Travell J, Rinzler SH. Pain syndromes of the chest muscles; resemblance to effort angina and myocardial infarction, and relief by local block. Can Med Assoc J 1948;59:333-338. PMID: 18886698.


18. Travell J, Rinzler SH. The myofascial genesis of pain. Postgrad Med 1952;11:425-434. PMID: 14920327.


19. Muscolino JE. The muscle and bone palpation manual with trigger points, referral patterns and stretching. St. Louis: Elsevier/Mosby; 2009.
20. Ombregt L. A system of orthopaedic medicine. Edinburgh: Churchill Livingstone; 2013.
21. Kelly M. Some rules for the employment of local analgesia in the treatment of somatic pain. Med J Aust 1947;1:235-239. PMID: 20291539.


22. Long C. Myofascial pain syndromes. II. Syndromes of the head, neck and shoulder girdle. Henry Ford Hosp Med Bull 1956;4:22-28. PMID: 13318636.

23. Reynolds MD. Myofascial trigger point syndromes in the practice of rheumatology. Arch Phys Med Rehabil 1981;62:111-114. PMID: 7235894.

24. Criscuolo CM. Interventional approaches to the management of myofascial pain syndrome. Curr Pain Headache Rep 2001;5:407-411. PMID: 11560805.


25. Rivner MH. The neurophysiology of myofascial pain syndrome. Curr Pain Headache Rep 2001;5:432-440. PMID: 11560808.


26. Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point: the importance of the local twitch response. Am J Phys Med Rehabil 1994;73:256-263. PMID: 8043247.


27. Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol 2004;14:95-107. PMID: 14759755.


28. Zhuang X, Tan S, Huang Q. Understanding of myofascial trigger points. Chin Med J (Engl) 2014;127:4271-4277. PMID: 25533832.


29. Ito N. Electromyographic study of shoulder joint. Nihon Seikeigeka Gakkai Zasshi 1980;54:1529-1540. PMID: 7229462.

Fig. 1
Proper position during the injection procedure. (A) The patient is positioned prone with the affected arm extended, internally rotated, and adducted. The patient then tries to reach the thoracic spine. In this position, the border of scapular bone moves away from other surrounding tissues and the border of the infraspinatus muscle is easily observed. (B) The probe is positioned on the most tender point to show the transverse image of the infraspinatus muscle, and an out-of-plane approach is used for the needle.
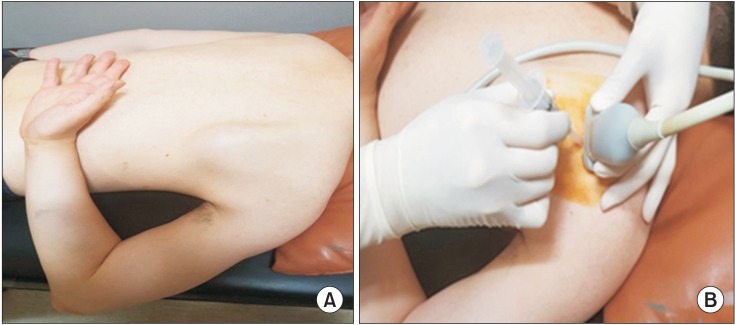
Fig. 2
Ultrasound images of the infraspinatus muscle. The ultrasound transducer was placed separately in the images shown in panels (A), (B), and (C). Transverse scan shows the infraspinatus, deltoid, and teres minor muscles. Isp, infraspinatus; Tm, teres minor; D, deltoid.
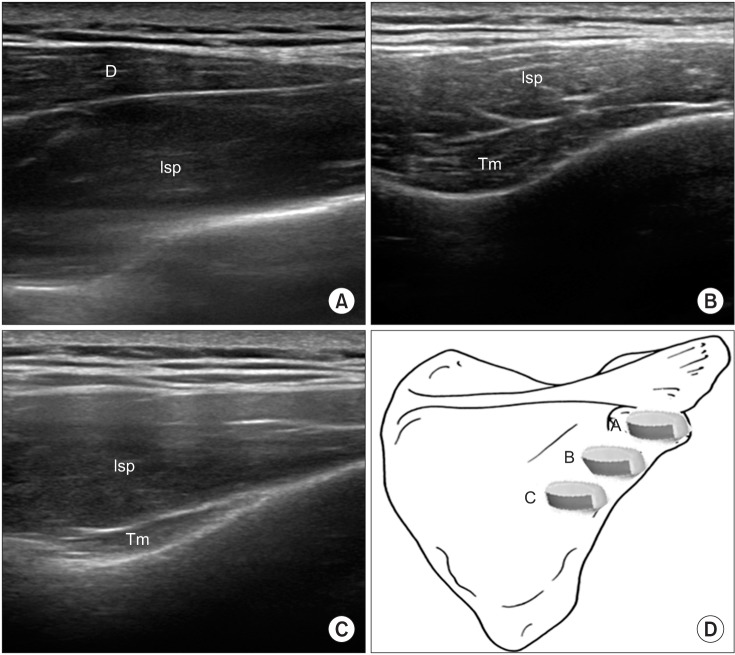
Fig. 3
Distribution of myofascial trigger points (MTrPs). MTrPs measurement sites (circles) and needling sites (at the center of each circle). The triangular infraspinatus muscle is divided into 10 subareas on each side of the shoulder. Values are presented as numbers of MTrPs on the painful side (%).
- TOOLS
-
METRICS

- Related articles in ARM
-
The MMPI Characteristics of Low Back and Cervical Pain Patients1995 September;19(3)
Shoulder Function Test in Myofascial Pain Syndrome1995 June;19(2)
Characteristics of Sympathetic Skin Response in Healthy Humans.1997 April;21(2)
Psychological Investigation in Myofascial Pain Syndrome Patients.1997 October;21(5)
Chronic Myofascial Pain Syndrome and Postherpetic Neuralgia.1998 April;22(2)