The Differences in Cardiac Rehabilitation Outcomes by Age in Myocardial Infarction: A Preliminary Study
Article information
Abstract
Objective
To determine the age-related changes in cardiac rehabilitation (CR) outcomes, which includes hemodynamic and metabolic factors, in patients with myocardial infarction (MI).
Methods
CR was administered for 8 weeks to 32 men (mean age, 54.0±8.8 years) who underwent percutaneous coronary intervention for acute MI between July 2012 and January 2016. The exercise tolerance tests were performed before and after the CR. The results were stratified based on a cut-off age of 55 years.
Results
In the whole patient group, the hemodynamic variables such as the resting heart rate (HRrest), systolic blood pressure (SBPrest), submaximal HR (HRsubmax), SBP (SBPsubmax), and rate pressure product (RPPsubmax) significantly decreased and the maximal HR (HRmax) and RPP (RPPmax) significantly increased. All metabolic variables displayed significant improvement, to include maximal oxygen consumption (VO2max) and ventilation (VEmax), anaerobic threshold (AT), and the maximal oxygen pulse (O2pulsemax). However, upon stratification by age, those who were younger than 55 years of age exhibited significant changes only in the HRrest and RPPsubmax and those aged 55 years old or greater displayed significant changes in all hemodynamic variables except diastolic BP. Both groups displayed significant increases in the VO2max, VEmax, and AT; the older group also exhibited a significant increase in O2pulsemax. The magnitude of the changes in the hemodynamic and metabolic variables before and after CR, based on age, did not differ between the groups; although, it tended to be greater among the older participants of this study's sample.
Conclusion
Because the older participants tended to show greater hemodynamic and metabolic changes due to CR, a more aggressive CR program must be administered to elderly patients with MI.
INTRODUCTION
Cardiac rehabilitation (CR) is known to improve patients' exercise capacity and health-related quality of life, which reduce the risk factors and mortality rates associated with cardiac diseases [1]. From the hemodynamics perspective, CR increases the tolerance to physical events associated with daily living activities by decreasing the myocardial oxygen consumption at submaximal workload [2]; in terms of metabolism, it is known to improve endurance by increasing the maximal oxygen consumption (VO2max) and anaerobic threshold (AT) [3].
Despite these advantages, the average participation rate in CR is only 30% in the United States [4]. The participation rate in CR decreases particularly with age, which could be attributed to the accompanying comorbidities that interrupt participation in CR in the elderly and the biases of physicians who believe that CR would be less effective in the elderly population [5].
A study demonstrated that aerobic exercise significantly improved exercise capacity regardless of age [6]. Another study showed that CR resulted in significant improvement in exercise capacity across all ages; although the magnitude of improvement was small among those who were over 75 years old [7].
However, these studies compared changes only in quality of life indices and overall exercise capacity such as VO2max and metabolic equivalent (MET) after CR [67]. Moreover, most studies on detailed hemodynamic and metabolic changes included only healthy adults without any underlying diseases [89]. Studies that included patients with coronary artery disease were limited by a high proportion of the elderly patients failing to achieve their true maximal cardiopulmonary limitation, which makes it difficult to accurately compare CR outcomes based on age [10].
Therefore, this study implemented a phase 2 CR therapy for patients who underwent percutaneous coronary intervention (PCI) to examine the age-related differences in CR outcomes in terms of hemodynamic and metabolic variables.
MATERIALS AND METHODS
Patients
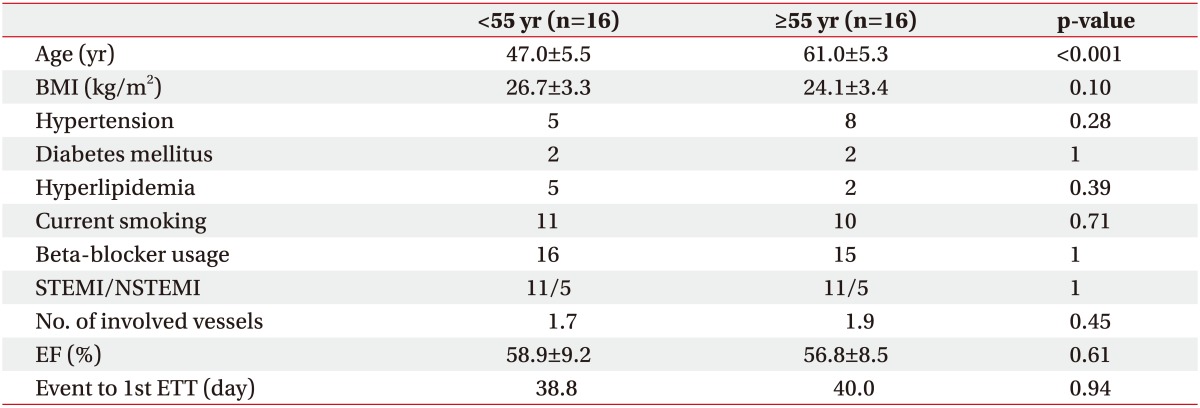
From July 2012 to January 2016, a total of 597 patients who underwent PCI with acute myocardial infarction (AMI) in Chungbuk National University Hospital were referred to the outpatient phase 2 cardiac rehabilitation program. Among them, 103 patients (90 men and 13 women) who did not have a contraindication to exercise testing based on the American Heart Association's (AHA) guidelines [11], agreed to the CR program, and underwent an initial exercise tolerance test (ETT) as the first step. Among them, 39 patients (32 men and 7 women) underwent supervised exercise CR at the hospital for more than 7 weeks during the rehabilitation period of 8 weeks and received a follow-up ETT. The female patients were excluded from this study. A retrospective chart review was conducted to analyze the hemodynamic and metabolic changes according to age after CR in the 32 male patients. They were divided into two groups (16 each) based on a cut-off age of 55 years. The chosen cutoff age was appropriate since hemodynamic responses with exercise differ with age, such as decreased cardiac output and increased pulmonary artery pressure among those older than 55 years [1213].
With respect to the risk factors of AMI, a self-report questionnaire was administered to collect information on diabetes mellitus, hypertension, and smoking status among those older than 55 years old. Anthropometric measurements, such as the body mass index (BMI), were gathered at the time of disease onset.
Among the potential patients for CR, those with contraindications for the ETT (such as untreated life-threatening cardiac arrhythmia or uncontrolled hypertension, symptomatic aortic stenosis, and acute heart failure), an acute systemic illness (e.g., musculoskeletal diseases, such as lower extremity amputation or arthralgia, and history of cerebrovascular disease) or respiratory diseases that can interfere with ETT [11] were excluded. The use of electronic medical records in the present study was approved by the appropriate Institutional Review Board of Chungbuk National University Hospital (IRB No. 2017-02-004).
Study methods
The patients underwent a symptom-limited graded ETT, based on the Modified Bruce Protocol, for an average of 39.4 days after the onset of AMI and subsequently participated in an 8-week CR program that focused on aerobic exercise. After completing the program, the patients underwent another ETT. During testing, if any of the early termination criteria by AHA [11] were met, testing was terminated.
With respect to examination tools, the Quinton Q-Stress (Mortara Instrument, Milwaukee, WI, USA) was used for real-time electrocardiogram (ECG) monitoring and Sun-Tech 247 BP device (SunTech Medical, Morrisville, NC, USA) was used for automatic blood pressure (BP) measurements. The Q-Stress TM55 (Mortara Instrument) was the type of treadmill used, and the TrueOne 2400 (Parvo Medics, Sandy, UT, USA) was used for respiratory gas analysis.
ECG monitoring and automatic BP measurements were used to measure resting heart rate (HRrest) and BP (BPrest), submaximal (the third exercise stage) HR (HRsubmax) and BP (BPsubmax), and maximal HR (HRmax) and BP (BPmax). The respiratory gas analyzer was used to measure VO2max, maximal ventilation (VEmax), and AT. The maximal oxygen pulse (O2pulsemax) was obtained by dividing the VO2max by the HRmax.
The exercise intensity in the CR program was determined using the Karvonen formula [14] based on the HRrest and HRmax measured during the initial ETT. Based on the risk classification for exercise training by the American Association of Cardiovascular and Pulmonary Rehabilitation guidelines [15], the exercise intensity in the first week of CR was set as 65%, 60%, and 50% of the heart rate reserve for the low-, medium-, and high-risk groups, respectively. The exercise intensity was increased gradually, up to 85% by the 8th week. The exercise intensity was adjusted so that the perceived exertion during exercise did not exceed 12–14 points according to the Borg Rating of Perceived Exertion Scale.
A single session of CR exercise lasted 50 minutes, which consisted of 10 minutes of warm-up, 30 minutes of prescribed exercise using a treadmill and cycle ergometer, and 10 minutes of cool-down. During exercise, telemetry was used to monitor the patient's ECG and HR, while BP was measured before, during, and after the exercise session. Patients visited the hospital once a week for supervised CR exercise and were instructed to perform home-based aerobic exercise for more than 5 days per week. The exercise intensity was reset each week using the target heart rate measured by telemetry during the supervised CR exercise.
Statistical analysis
The between-group differences were tested using the Mann-Whitney test for continuous variables (such as age, BMI, number of involved vessels, and ejection fraction measured prior to CR) and the Pearson chi-square or Fisher exact test was used for non-continuous variables (such as hypertension and current smoking status). A paired t-test was used for the statistical analysis of hemodynamic and metabolic changes before and after CR in all patients; while the Wilcoxon signed-rank test was used to determine the statistical significance of hemodynamic and metabolic changes before and after CR in the two groups based on age.
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) ver. 24.0 software (IBM, Armonk, NY, USA). The statistical significance level was set at a p-value of less than 0.05.
RESULTS
General characteristics of patients
The mean patient age was 54.0±8.8 years (range, 34–76 years); while the mean age in the young and old groups were 47.0±5.5 and 61.0±5.3 years, respectively. Other variables apart from age that can affect exercise capacity, such as diabetes mellitus, hypertension, BMI, current smoking, beta-blocker usage, and ejection fraction [16], were not significantly different between the two groups (Table 1).
Hemodynamic and metabolic changes after CR in all patients
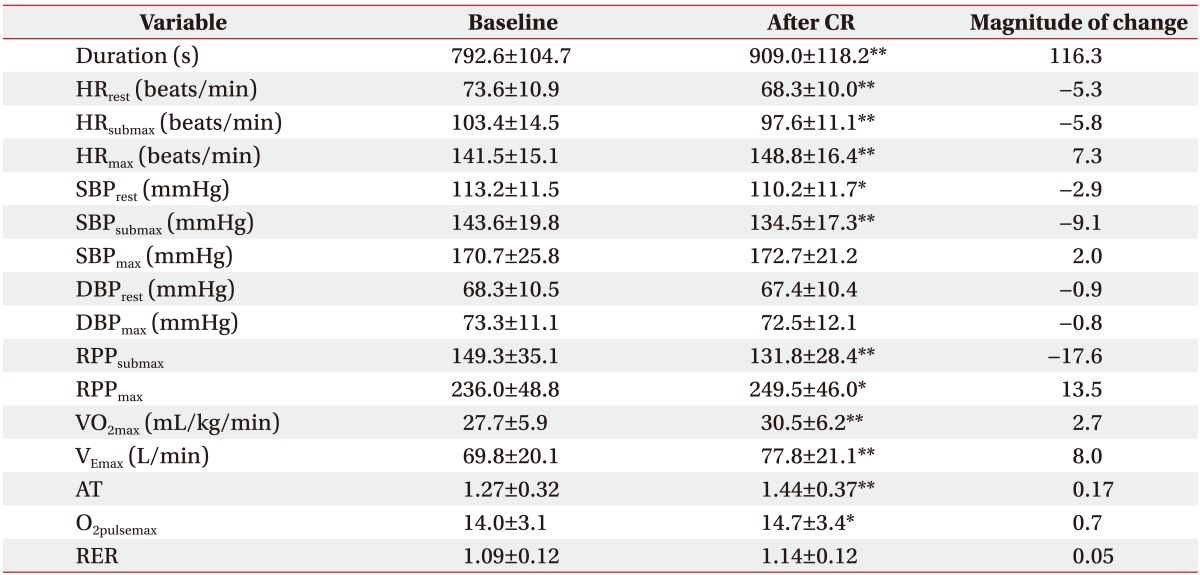
After the 8-week CR therapy, among the hemodynamic variables, exercise duration (+116.3 s), HRmax (+7.3 beats/min), and RPPmax (+13.5) exhibited significant increases; whereas HRrest (−5.3 beats/min), SBPrest (−2.9 mmHg), HRsubmax (−5.8 beats/min), SBPsubmax (−9.1 mmHg), and RPPsubmax (−17.6) displayed significant decreases.
With respect to the metabolic variables after CR, VO2max (+2.7 mL/kg/min), VEmax (+8.0 L/min), AT (+0.17), and O2pulsemax (+0.7) showed statistically significant increases (Table 2).
Change of HRmax after CR according to reaching maximal limitation
Among the results of the ETT performed before CR, the respiratory exchange ratio (RER), which is an indicator of whether a patient's exercise reached the patient's true maximal cardiopulmonary limitation [17], was used with a cut-off value of 1.0 to divide all patients into two groups. The magnitude of change in HRmax before and after CR was +13.3 beats/min in the RER <1.0 group (p<0.05); whereas, the RER ≥1.0 group displayed an increasing trend with +5.3 beats/min. However, the change was not statistically significant (Table 3). Moreover, when the patients were divided into two age groups with a cut-off age of 55 years, both groups exhibited a mean RER value of 1.0 or greater in the ETT performed before CR. The percentage of patients with a RER of 1.0 or greater did not differ between the two groups with an 81% represented in both the young and old groups (n=13 each). Even in the test after CR, the two groups exhibited no difference in this regard, at 94% in each group (n=15 each) (Table 4).
Hemodynamic and metabolic changes after CR by age group
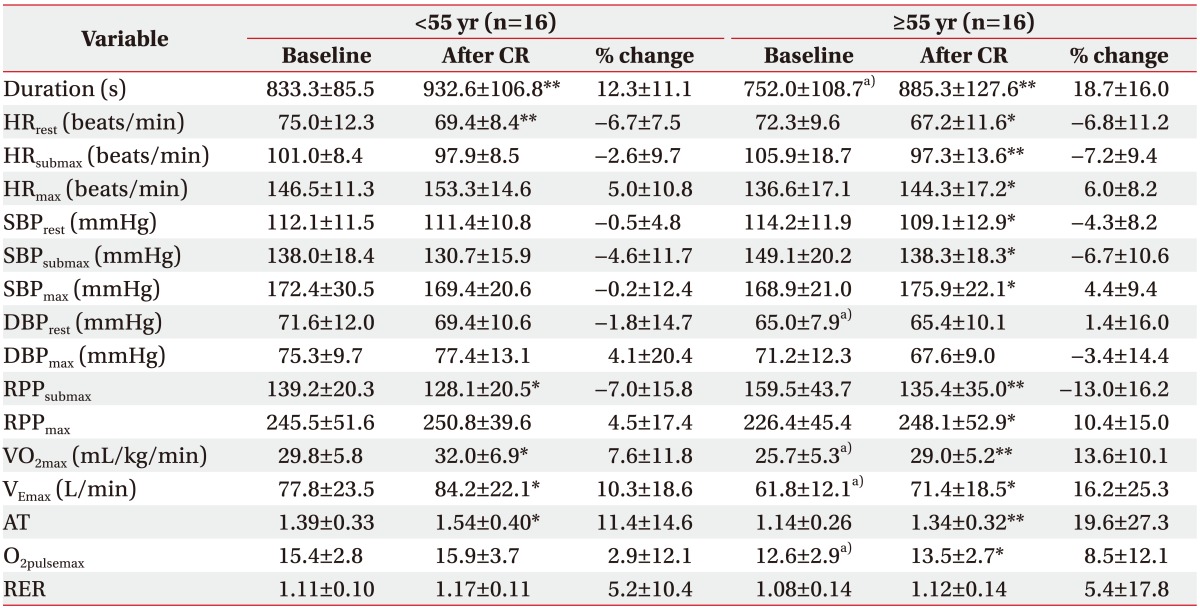
Before CR, the older group had a significantly shorter exercise duration (−81 s) and significantly lower VO2max (−4.1 mL/kg/min), VEmax (−16 L/min), and O2pulsemax (−2.8) than the young group. These results indicate a decreasing trend in overall exercise capacity with increases in age.
After the 8-week CR therapy in the older group, significant improvements were seen in all variables except for diastolic blood pressure (DBP); that is, significant increases were observed in exercise duration (+133.3 s), HRmax (+7.7 beats/min), SBPmax (+7.0 mmHg), and RPPmax (+21.7) and significant decreases were observed in HRrest (−5.1 beats/min), SBPrest (−5.1 mmHg), HRsubmax (−8.6 beats/min), SBPsubmax (−10.8 mmHg), and RPPsubmax (−24.1). However, in the young group, among the hemodynamic variables, a significant increase in exercise duration (+99.4 s) and significant decreases in HRrest (−5.6 beats/min) and RPPsubmax (−11.1) were evidenced. All other variables were not statistically significant.
With respect to metabolic variables, after CR, both groups demonstrated significant increases in VO2max, VEmax, and AT. However, only the older group had a significant increase in the O2pulsemax, from 12.6±2.9 before exercise to 13.5±2.7 after exercise (Table 4).
Comparison of hemodynamic and metabolic changes after CR between the young and the old groups
There were no statistically significant differences in the percent change of all hemodynamic variables from before to after CR in the two age groups. However, compared to the young group, the older group exhibited a higher percent change in HRsubmax (−2.6% vs. −7.2%), RPPsubmax (−7.0% vs. −13.0%), and RPPmax (4.5% vs. 10.4%). Furthermore, there were no significant differences in the percent change of all metabolic variables after CR in the two age groups. However, the older group experienced a higher percent change in VO2max (7.6% vs. 13.6%), AT (11.4% vs. 19.6%), and O2pulsemax (2.9% vs. 8.5%) (Table 4).
Cardiovascular complications during exercise
During the ETT and CR exercises, there were no abnormal ECG findings, such as sustained ventricular tachycardia. Furthermore, there were no major cardiac complications, such as abnormal hemodynamic response, MI, cardiac arrest, and syncope in both groups.
DISCUSSION
Improvement in exercise capacity with CR can be attributed to improved heart function, such as increases in stroke volume [18] and coronary perfusion due to improved endothelium-dependent coronary vasodilation [19], as well as increased peripheral oxygen extraction (such as increases in capillaries in peripheral skeletal muscles, myoglobin count, number and size of mitochondria and oxidase level) [3].
Administered CR to patients with coronary artery disease results in hemodynamic changes including decreased HR, BP, and RPP at rest and under the same exercise intensity, because of the physiological changes already mentioned. These changes in turn increased the exercise tolerance of daily living activities [20]. CR is also known to increase RPPmax upon angina onset through increased coronary perfusion [121]. In addition, metabolically, the VO2max increases, which is an important variable related to the patient's prognosis [3]. The VEmax increases, which indicates effective oxygen delivery to the tissues, carbon dioxide emission, and lactic acid neutralization [22]. In addition, AT, as a key predictor of endurance, increases because of the ability to perform higher intensity exercise without lactic acid accumulation [3]. Furthermore, the oxygen consumption per pulse at peak exercise intensity, expressed as O2pulsemax, reflects cardiovascular efficiency during exercise and is an important factor in predicting the prognosis of CAD patients similar to VO2max. O2pulsemax also increases with CR [23].
The findings of the current study with regard to changes in hemodynamic and metabolic variables after CR in the entire patient population are in line with previous studies (Table 2), except for the HRmax. The HRmax is determined by age, and therefore should display no change or decrease after aerobic exercise [24]. However, in the current study, it demonstrated an increasing pattern. When an initial ETT was performed in a deconditioning state for various reasons such as the medical condition before CR, the initial HRmax was measured lower than expected due to the failure of the patient reaching the maximal cardiopulmonary limitation. As such, the changes in HRmax may appear to have increased due to aerobic exercise since it improves in a deconditioning state after CR. In our study, only the RER >1.0 group exhibited a significant increase in the HRmax; which indicates that the results were affected by patients who failed to reach their true maximal limitation due to deconditioning prior to CR (Table 3).
However, conflicting results were found in the analysis with the patients divided into two groups based on age. Among the hemodynamic variables, the young group exhibited significant changes only in exercise duration, HRrest, and RPPsubmax; whereas, the older group showed significant changes in all hemodynamic variables except for DBP (Table 4). In particular, although the magnitude of change in RPPsubmax was not significant, it tended to be higher in the older group, which indicates that the elderly have a greater potential for performing higher intensity activities with the same RPP after CR. In addition, considering that there were no differences in the rate of reaching the true maximal cardiopulmonary limitation in the ETT between the two age groups, we speculate that the higher percent change of RPPmax in the older group could be attributed to increased myocardial oxygen delivery because of exercise training [19]. This outcome was also confirmed in a study by DeSouza et al. [25] that demonstrated that the endothelium-dependent vasodilation effect from exercise training was more pronounced among the elderly.
With respect to metabolic changes based on age, the young group showed a pattern of increasing O2pulsemax, but this change was not statistically significant. Whereas, the older group experienced significant increases in all metabolic variables, including the O2pulsemax (Table 4). Although there were no statistically significant differences in the magnitude of change between the young and old groups, the older group displayed a greater margin of change. As in the case of the hemodynamic variables, this outcome indicates that although the older group showed lower metabolic variables, the effects of CR were equivalent to or higher compared with those in the young group.
In healthy adults, an increase in age is associated with a lower ejection fraction, HRmax, and arteriovenous oxygen difference, as well as reduced blood flow to and capillary density of skeletal muscles during peak exercise intensity, which results in a gradual decrease in exercise capacity [26]. However, several studies have shown that despite these physiological disadvantages, the elderly exhibited comparable or even greater improvement in exercise capacity than the younger populations [67], which aligns with the results of this study. In a previous study, compared to the young, CR was perceived less effective in the elderly because the elderly tended to have diminished exercise capacity and accompanying diseases that can interfere with exercise [5]. However, the findings of this study confirm that CR can generate greater hemodynamic and metabolic changes in the elderly than in the younger populations. Therefore, it is necessary to actively encourage older patients to participate in CR.
The present study had some limitations. First, the sample size was small, and because all of the patients were males, the findings may not be generalizable. Therefore, future studies that include a greater number of patients for analysis by age and sex are necessary. Second, only the short-term effects of CR were assessed, and only the results immediately before and after CR were compared. Therefore, studies with a long-term follow-up are necessary to assess the long-term effects of CR. Finally, this study included only patients who underwent PCI after AMI. Therefore, these findings may not be applicable to patients who underwent other procedures, such as coronary artery bypass surgery, or to patients with other heart diseases, such as chronic heart failure and heart valve disease.
In conclusion, those who were 55 years and older had significantly better hemodynamic and metabolic improvements after CR for AMI than the younger populations. Therefore, the CR program must be actively recommended to older patients with MI.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.