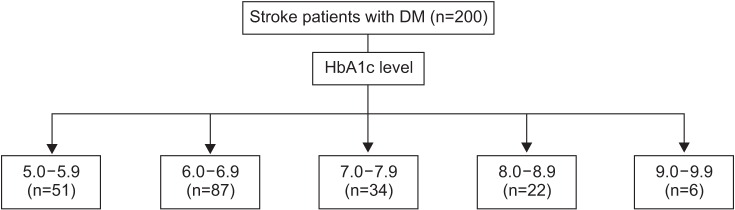
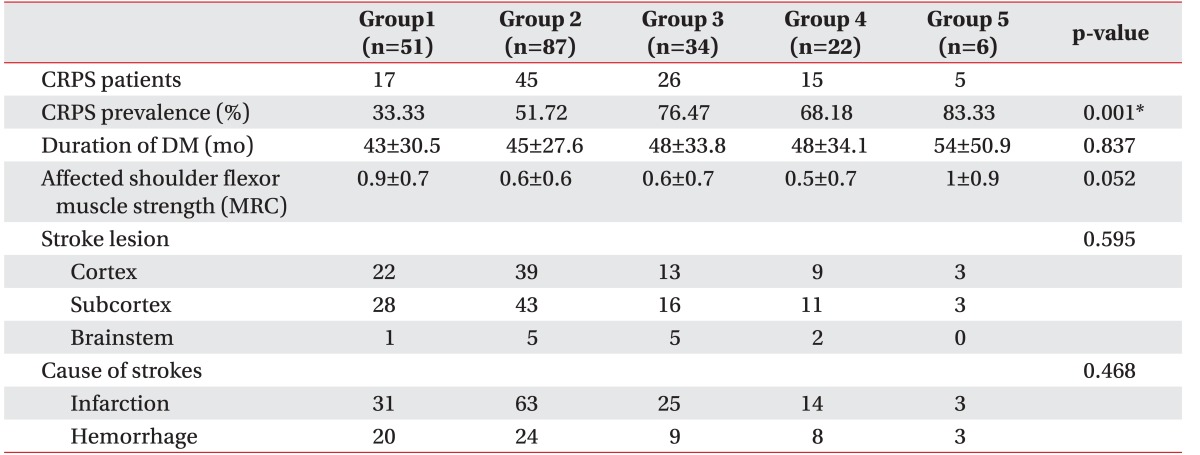
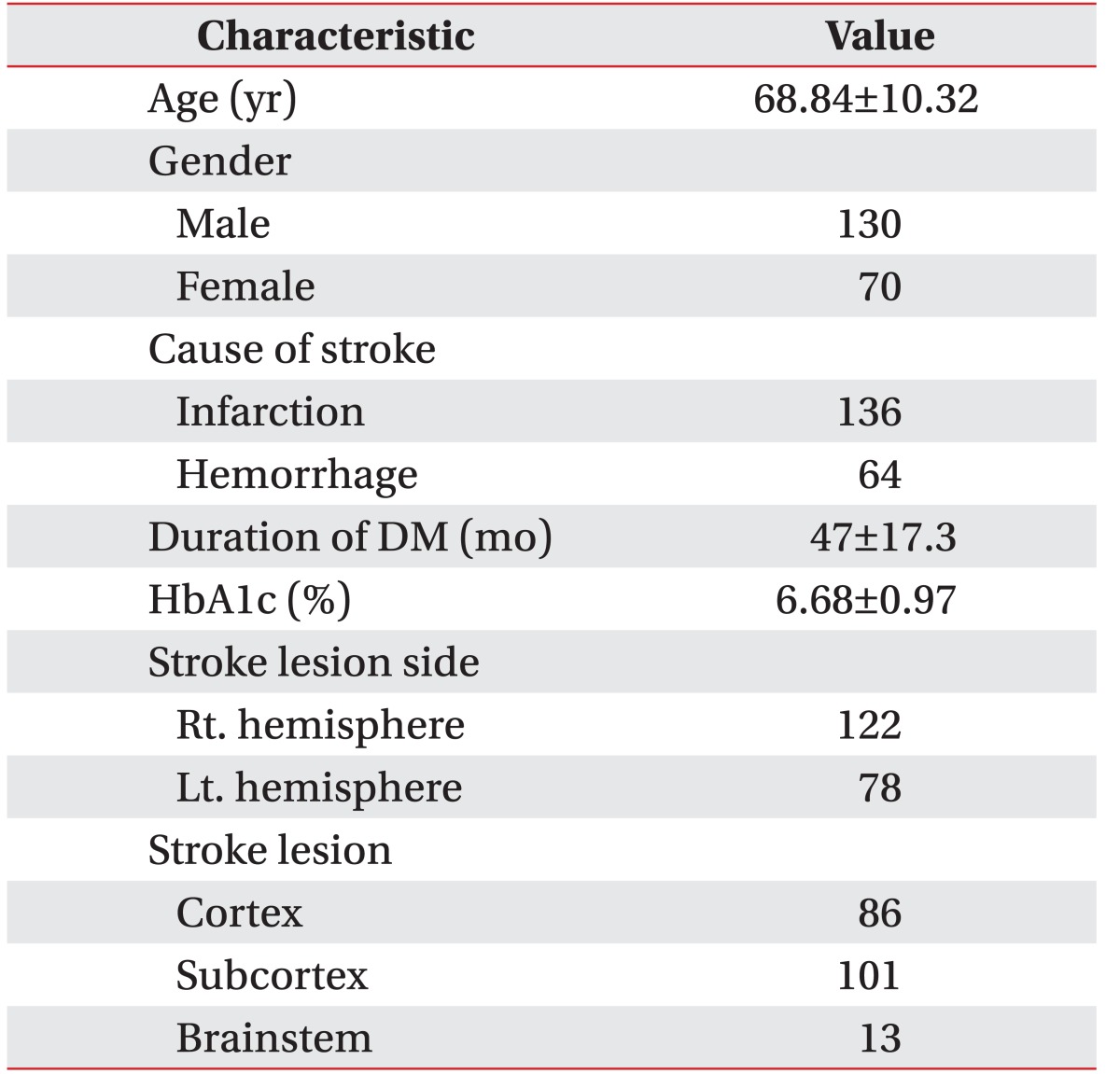
1. Stanton-Hicks M, Janig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain 1995;63:127-133. PMID:
8577483.


2. Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Seattle: IASP Press; 1994.
3. Pertoldi S, Di Benedetto P. Shoulder-hand syndrome after stroke: a complex regional pain syndrome. Eura Medicophys 2005;41:283-292. PMID:
16474282.

4. Tepperman PS, Greyson ND, Hilbert L, Jimenez J, Williams JI. Reflex sympathetic dystrophy in hemiplegia. Arch Phys Med Rehabil 1984;65:442-447. PMID:
6466074.

5. Cheng PT, Hong CZ. Prediction of reflex sympathetic dystrophy in hemiplegic patients by electromyographic study. Stroke 1995;26:2277-2280. PMID:
7491650.


6. Daviet JC, Preux PM, Salle JY, Lebreton F, Munoz M, Dudognon P, et al. Clinical factors in the prognosis of complex regional pain syndrome type I after stroke: a prospective study. Am J Phys Med Rehabil 2002;81:34-39. PMID:
11807329.


7. Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol 2011;10:637-648. PMID:
21683929.



8. Wyatt LH, Ferrance RJ. The musculoskeletal effects of diabetes mellitus. J Can Chiropr Assoc 2006;50:43-50. PMID:
17549168.


9. Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 1998;41:1241-1248. PMID:
9794114.


10. Malkani S, Mordes JP. Implications of using hemoglobin A1C for diagnosing diabetes mellitus. Am J Med 2011;124:395-401. PMID:
21531226.



11. Graf RJ, Halter JB, Pfeifer MA, Halar E, Brozovich F, Porte D Jr. Glycemic control and nerve conduction abnormalities in non-insulin-dependent diabetic subjects. Ann Intern Med 1981;94:307-311. PMID:
7013592.


12. Marshall AT, Crisp AJ. Reflex sympathetic dystrophy. Rheumatology (Oxford) 2000;39:692-695. PMID:
10908684.



13. Kim RP. The musculoskeletal complications of diabetes. Curr Diab Rep 2002;2:49-52. PMID:
12643122.


14. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med 2007;8:326-331. PMID:
17610454.



15. Stanton-Hicks MD, Burton AW, Bruehl SP, Carr DB, Harden RN, Hassenbusch SJ, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract 2002;2:1-16. PMID:
17134466.


16. Calder JS, Holten I, McAllister RM. Evidence for immune system involvement in reflex sympathetic dystrophy. J Hand Surg Br 1998;23:147-150. PMID:
9607647.


17. van der Laan L, Goris RJ. Reflex sympathetic dystrophy: an exaggerated regional inflammatory response. Hand Clin 1997;13:373-385. PMID:
9279543.

18. Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 1998;30:5-11. PMID:
9457475.


19. Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology 2001;57:2179-2184. PMID:
11756594.


20. Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain 2006;22:235-239. PMID:
16514322.


21. Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett 2004;91:147-154. PMID:
15019283.


23. Uceyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain 2007;132:195-205. PMID:
17890011.


24. Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators Inflamm 2008;2008:469439PMID:
18596918.


25. Parkitny L, McAuley JH, Di Pietro F, Stanton TR, O'Connell NE, Marinus J, et al. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology 2013;80:106-117. PMID:
23267031.



26. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813-823. PMID:
14988310.


27. Crook MA, Tutt P, Pickup JC. Elevated serum sialic acid concentration in NIDDM and its relationship to blood pressure and retinopathy. Diabetes Care 1993;16:57-60. PMID:
8422833.


28. Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine 2012;57:136-142. PMID:
22035595.


29. Alexandraki KI, Piperi C, Ziakas PD, Apostolopoulos NV, Makrilakis K, Syriou V, et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol 2008;28:314-321. PMID:
18224429.


30. Bastard JP, Pieroni L, Hainque B. Relationship between plasma plasminogen activator inhibitor 1 and insulin resistance. Diabetes Metab Res Rev 2000;16:192-201. PMID:
10867719.


31. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98-107. PMID:
21233852.



32. Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 2000;67:291-300. PMID:
10983873.


33. Dandona P, Aljada A, Chaudhuri A, Bandyopadhyay A. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes. J Clin Endocrinol Metab 2003;88:2422-2429. PMID:
12788837.


34. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet 1993;342:1012-1016. PMID:
8105263.


35. Kozin F. Reflex sympathetic dystrophy syndrome: a review. Clin Exp Rheumatol 1992;10:401-409. PMID:
1395224.

36. Schwartzman RJ, McLellan TL. Reflex sympathetic dystrophy: a review. Arch Neurol 1987;44:555-561. PMID:
3495254.


37. Shelton RM, Lewis CW. Reflex sympathetic dystrophy: a review. J Am Acad Dermatol 1990;22:513-520. PMID:
2097997.