Clinical Findings of Asymptomatic Carpal Tunnel Syndrome in Patients With Diabetes Mellitus
Article information
Abstract
Objective
To evaluate the clinical differences between patients with diabetes mellitus (DM) who have asymptomatic carpal tunnel syndrome (CTS) and those who have symptomatic CTS.
Methods
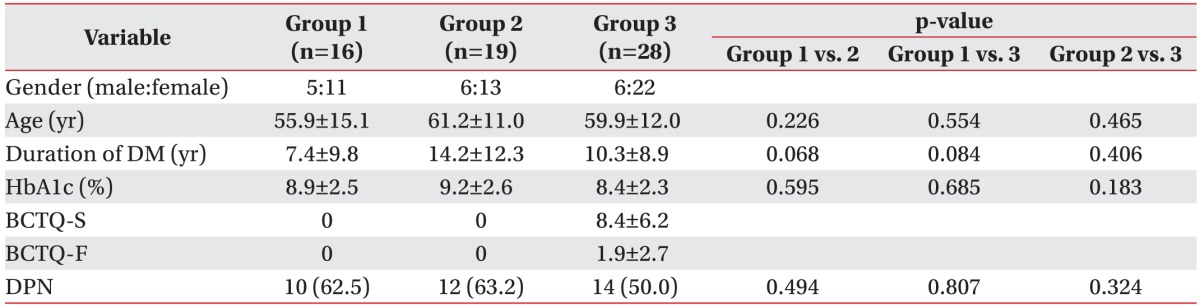
Sixty-three patients with DM were assessed using the Boston Carpal Tunnel Questionnaire (BCTQ), nerve conduction studies (NCS), and ultrasonographic evaluation of the cross-sectional area (CSA) of the median nerve. According to the BCTQ responses and NCS results, the patients were divided into the following three groups: group 1 (n=16), in which NCS results did not reveal CTS; group 2 (n=19), in which NCS results revealed CTS but the group scored 0 points on the BCTQ (asymptomatic); and group 3 (n=28), in which NCS results revealed CTS and the group scored >1 point on the BCTQ (symptomatic). The clinical findings, NCS results, and CSA of the median nerve were compared among the three groups.
Results
There were no significant differences in age, DM duration, glycated hemoglobin levels, and presence of diabetic polyneuropathy among the three groups. The peak latency of the median sensory nerve action potential was significantly shorter in group 1 than in groups 2 and 3 (p<0.001); however, no difference was observed between groups 2 and 3. CSA of the median nerve at the carpal tunnel in group 2 was significantly larger than that in group 1 and smaller than that in group 3 (p<0.05).
Conclusion
The results of our study suggest that the symptoms of CTS in patients with diabetes are related to CSA of the median nerve, which is consistent with swelling of the nerve.
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most commonly reported mononeuropathy in patients with diabetes mellitus (DM). Although the prevalence of CTS is 2%–3% among the general population [12], its prevalence is approximately 7%–16% in patients with diabetes [34]. Previous studies have reported DM as a risk factor for CTS [56] and identified relationships between the prevalence of CTS and level of hemoglobin A1c (HbA1c), duration of DM, and microvascular complications [789]. However, 14%–25% of diabetic patients with CTS do not have clinical symptoms, and it is unclear why this subpopulation does not exhibit the typical signs of nerve entrapment [10].
The diagnosis of CTS is usually based on clinical and electrophysiological findings. Although a nerve conduction study (NCS) is highly specific for the diagnosis of CTS, it can be difficult to differentiate CTS in patients with diabetes and coexisting diabetic polyneuropathy. In recent years, the accuracy of high-resolution diagnostic ultrasonographic equipment has greatly improved. Consequently, the use of ultrasonography for the diagnosis of CTS has been increasing. The most common ultrasonographic findings in patients with nerve entrapment syndrome are sudden flattening of nerves in the zone of entrapment and swelling of the nerve at the proximal edge of the zone of compression [2]. In previous studies, a significant increase in the cross-sectional area (CSA) of the median nerve at the carpal tunnel was the most consistent sonographic finding of CTS [1112].
In order to investigate the clinical factors associated with the development of symptoms in diabetic patients with CTS, we compared the clinical characteristics and findings, including NCS results and ultrasonographic findings, between diabetic patients with symptomatic CTS and those with asymptomatic CTS.
MATERIALS AND METHODS
Participants
The present study was a retrospective investigation of patients with DM greater than 20 years of age who underwent NCS at the electrodiagnostic clinic of Incheon St. Mary's Hospital from June 2011 to November 2014. This study was approved by the Institutional Review Board.
We excluded the patients who had previously undergone surgery for CTS; who had a previous history of trauma and had been diagnosed with fractures of the wrist; who had been diagnosed with cervical radiculopathy, brachial plexopathy, or a focal peripheral neuropathy other than CTS involving the upper extremities; who had medical diseases that could evoke peripheral polyneuropathies other than DM, such as chronic renal insufficiency, chronic alcoholism, toxin exposure, or hypothyroidism. We included a total of 63 dominant hands of diabetic patients, and all patients completed the Boston Carpal Tunnel Questionnaire (BCTQ) and underwent NCS and ultrasonographic evaluation of the median nerve CSA at the carpal tunnel on the same day.
On the basis of the results of these tests, the patients were classified into the following three groups: group 1 (n=16), in which NCS results did not reveal CTS; group 2 (n=19), in which NCS results revealed CTS but the group scored 0 points on the BCTQ; and group 3 (n=28), in which NCS results revealed CTS and the group scored >1 point on the BCTQ.
Clinical scale
Prior to the electrodiagnostic test, a physiatrist administered the BCTQ [13]. This is a self-report scale that assesses the degree of symptom severity and upper extremity function on the basis of the patient's responses according to symptom and functional scale scores. Asymptomatic CTS was defined as a score of 0 points, and symptomatic CTS was defined as a score of >1 point following summation of symptom and functional scale scores. The validity and reliability of the BCTQ for the detection of symptom severity and functional status of CTS patients have been confirmed in many studies [1415161718]. We used the Korean version of the BCTQ, which has also been shown to have good validity and reliability [19].
Nerve conduction study
All subjects underwent NCS using an electromyography device (VIASYS Healthcare, Surrey, UK) and surface electrodes (VIASYS Healthcare). The skin temperature of the patients was measured prior to the test, and the temperature of each hand was maintained above 32℃. Motor conduction studies were performed on the median, ulnar, peroneal, and tibial nerves. F-waves were recorded from the median and tibial nerves. Sensory nerve action potentials (SNAPs) were obtained from the median, ulnar, and sural nerves. The median motor response was recorded from the abductor pollicis brevis muscle using supramaximal stimulation 7 cm from the active electrode; the onset latency and baseline-to-peak amplitude were then measured. The median sensory response was measured antidromically in the third digit, and stimulation was applied at two points: 7 and 14 cm proximal to the active electrode. The ulnar motor response was recorded from the abductor digiti minimi muscle using stimulation 7 cm from the active electrode. The ulnar sensory response was measured antidromically in the fifth digit, and stimulation was applied to the wrist 14 cm proximal to the active electrode. A sensory comparative study of the ring finger was performed when the peak latency of the median SNAP at the wrist was delayed. The electrode was placed over the fourth digit, and stimulations were applied to the median and ulnar sides of the wrist 14 cm proximal to the active electrode for each position. Bilateral superficial peroneal, sural, deep peroneal, and tibial nerves were also assessed for making the diagnosis of diabetic polyneuropathy (DPN). Superficial peroneal and sural nerves were antidromically recorded 14 cm distal to the stimulated sites.
CTS was diagnosed according to the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation, practice parameters for electrodiagnostic studies in CTS: summary statement [20]. CTS was diagnosed in patients if they met the following criteria: (1) the peak latency of the median SNAP at the wrist (14 cm proximal to the active electrode) was delayed >3.6 ms; (2) the median-to-ulnar peak latency difference at the ring finger (14 cm proximal to the active electrode) was >0.5 ms; and (3) the wrist-to-palm latency difference was greater than the palm-to-digit latency difference in the median sensory nerve. The diagnosis of DPN was based on the Diabetes Control and Complications Trial [21]. All reference values were based on Dumitru et al.'s description [22].
Ultrasonography
An experienced Korean physician who was board certified in musculoskeletal ultrasound medicine performed the ultrasound examination using an HD11 XE device (Philips, Bothell, WA, USA) and a 7- to 12-MHz line-array transducer. The physician was blinded to the BCTQ scores and electrodiagnostic results. Ultrasonography was performed while the subject was lying supine with the wrist resting in a neutral position with the palm facing upwards. CSA of the median nerve was measured at the level of the pisiform bone using a direct tracing method within the echogenic boundary of the nerve. If the patient had a bifid median nerve, the summated CSA value of the nerves was used. CSA of the median nerve in each hand was estimated twice, and the mean value was recorded.
Statistical analyses
All statistical analyses were performed using SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Normality of the sample was tested by applying the Shapiro-Wilk test. The age and duration of DM were assessed using the Kruskal-Wallis test. HbA1c and the results of NCS were assessed using one-way analysis of variance (ANOVA). Comparisons of the CSA of the median nerve at the carpal tunnel among the three groups were analyzed by Mann-Whitney U test after Kruskal-Wallis test. The rate of DPN was analyzed using the chi-square test, and a p-value <0.05 was considered to indicate statistical significance.
RESULTS
A total of 63 dominant hands were included in this study: 9 left hands (14.3%) and 54 right hands (85.7%). Of the 63 subjects, 17 were male (27.0%) and 46 were female (73.0%). The mean age was 59 years (range, 29–85 years). The basic characteristics and BCTQ scores of the groups are summarized in Table 1. Multiple comparisons of the variables revealed that the mean age, mean duration of DM, mean value of HbA1c, and rate of coexistence of DPN were not significantly different among the three groups.
The mean peak latency of the median SNAP was significantly shorter in group 1 than in groups 2 and 3 (group 1, 3.5±0.6 ms; group 2, 4.6±0.6 ms; group 3, 5.0±1.0 ms). There was no difference in the mean peak latency of the median SNAP between groups 2 and 3. Similarly, the mean onset latency of the median compound motor action potential (CMAP) was significantly shorter in group 1 than in groups 2 and 3 (group 1, 3.4±0.7 ms; group 2, 4.5±0.8 ms; group 3, 4.9±0.9 ms). There was no difference in the mean onset latency of the median CMAP between groups 2 and 3. However, the mean peak latency of the ulnar SNAP and the onset latency of the ulnar CMAP were not significantly different among the three groups. The detailed electrodiagnostic findings are listed in Table 2.
The mean CSA of the median nerve at the carpal tunnel in groups 1, 2, and 3 was 8.6, 12.5, and 15.5 mm2, respectively. The mean CSA of the median nerve in group 2 was significantly larger than that in group 1 (p<0.001), but it was significantly smaller than that in group 3 (p=0.007) (Table 2).
DISCUSSION
In our study, the mean CSA of the median nerve was larger in diabetic patients with symptomatic CTS than in those with asymptomatic CTS. This is the first study to compare the NCS results and ultrasonographic findings according to the presence of CTS symptoms in patients with diabetes.
Several previous studies have reported that CTS is more prevalent in the population with diabetes [34] and DM is a risk factor for CTS [56]. The increased risk of CTS in patients with diabetes has been attributed to several different etiologies. The median nerves in patients with diabetes are vulnerable to focal compression due to endoneurial ischemia [9]. Additionally, increased end products of advanced glycation and myofibroblasts in the connective tissue of the flexor tendon result in synovitis and a thickened flexor retinaculum, which eventually makes the carpal tunnel narrow [232425]. However, CTS does not always cause clinical symptoms in patients with diabetes. Approximately 14%–25% of diabetic patients with CTS do not exhibit clinical symptoms, and it is unclear why this subpopulation does not display the symptoms of CTS.
Few studies on the clinical characteristics and outcomes of asymptomatic CTS have been reported. Kim et al. [25] reported that 6.8% of patients with diabetes had asymptomatic electrophysiological CTS and investigated whether the cause of asymptomatic CTS in patients with diabetes was a manifestation of early DPN or an entrapment neuropathy itself. They found that conduction delay in the finger-to-wrist segment was more remarkable in the median nerve than in the ulnar nerve. These results suggest that asymptomatic CTS in patients with diabetes is related to an increased vulnerability to the entrapment of the median nerve rather than to early DPN. Similarly, in our study, there were no differences in the DPN and NCS findings of the ulnar nerve among the three groups.
A study that followed patients with asymptomatic CTS for 11 years found that the probability of developing symptomatic CTS was correlated with differences in the maximum latency during the initial median NCS [26]. Another study reported that 23% of asymptomatic workers with abnormal median sensory nerve conduction developed symptomatic CTS after an average of 70 months [27]. Although these studies did not specifically evaluate patients with diabetes, their findings indicate that a close observation of asymptomatic CTS patients with diabetes is required.
The putative mechanism of the pathogenesis of asymptomatic CTS in patients with diabetes is an increased sensory threshold [2528]. The density of intraepidermal nerve fibers in the skin of patients with type 2 diabetes has been shown to be negatively associated with the duration of diabetes. Patients with lower density values exhibited higher sensory thresholds [29]. A rodent study found that, compared with subjects with a shorter disease duration, subjects with a longer duration of diabetes tended to exhibit insensate neuropathy rather than painful neuropathy [30]. Thus, there is evidence that the sensory threshold can increase in patients with diabetes who have had the disease for a long duration.
In our study, although there were no significant differences in the DM duration and HbA1c values among the three groups, we observed tendencies toward increased DM duration and elevated HbA1c values in the asymptomatic group than in the symptomatic group. These results are believed to be associated with the small sample size used in the present study; therefore, further studies with more patients are required.
CTS patients without diabetes have an increased CSA of the median nerve that is positively correlated with their electrodiagnostic severity [331]. Similarly, our results showed that the mean peak latency of the median SNAP and mean onset latency of the median CMAP tend to increase in symptomatic patients, although the increase was not significant in the statistical analysis. CSA of the median nerve was significantly increased in symptomatic patients. Because the nerve swelling associated with increasing water content seems to be correlated with a cascade of events that ultimately lead to axonal loss [32], it is possible that CSA of the median nerve is correlated with the severity of CTS.
There were several limitations in the present study. First, the sample size in each group was small. Larger sample sizes may yield more reliable results and greater statistical power. Second, we did not consider the body mass index of the individuals, a factor that can affect CSA of the median nerve. Third, CSA of the median nerve at the proximal forearm and CSA of the ulnar nerve were not measured. Comparison of these values may help to evaluate the swelling of the median nerve more precisely.
Our results for the ultrasonographic findings of the median nerve CSA among the groups suggested that the degree of median nerve swelling may be related to the occurrence of CTS symptoms in patients with diabetes. Therefore, regular ultrasonographic follow-up might be helpful for the prediction of symptom development in patients with diabetes who have been electrophysiologically diagnosed as having CTS but who do not display any symptoms. Further studies with larger sample sizes and significant follow-up periods are required to understand the mechanisms of asymptomatic CTS in patients with diabetes. Such studies may contribute to a better understanding of the underlying pathophysiology of CTS in these patients.
In conclusion, this study demonstrates that the mean CSA of the median nerve at the carpal tunnel was significantly larger in diabetic patients with symptomatic CTS than in diabetic patients with asymptomatic CTS. Our results suggest that the symptoms of CTS in patients with diabetes may be related to the swelling of the median nerve rather than to the duration of DM, glycemic control, coexistence of DPN, and severity of the values in NCS.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.