Correlation Between the Severity of Diabetic Peripheral Polyneuropathy and Glycosylated Hemoglobin Levels: A Quantitative Study
Article information
Abstract
Objective
To investigate risk factors for diabetic peripheral polyneuropathy and their correlation with the quantified severity of nerve dysfunction in patients with diabetes mellitus (DM).
Methods
A total of 187 diabetic patients with clinically suspected polyneuropathy (PN) were subclassified into 2 groups according to electrodiagnostic testing: a DM-PN group of 153 diabetic patients without electrophysiological abnormality and a DM+PN group of 34 diabetic patients with polyneuropathy. For all patients, age, sex, height, weight, duration of DM, and plasma glycosylated hemoglobin (HbA1c) level were comparatively investigated. A composite score was introduced to quantitatively analyze the results of the nerve conduction studies. Logistic regression analysis and multiple regression analysis were used to evaluate correlations between significant risk factors and severity of diabetic polyneuropathy.
Results
The DM+PN group showed a significantly higher HbA1c level and composite score, as compared with the DM-PN group. Increased HbA1c level and old age were significant predictive factors for polyneuropathy in diabetic patients (odds ratio=5.233 and 4.745, respectively). In the multiple linear regression model, HbA1c and age showed a significant positive association with composite score, in order (β=1.560 and 0.253, respectively).
Conclusion
Increased HbA1c level indicative of a state of chronic hyperglycemia was a risk factor for polyneuropathy in diabetic patients and a quantitative measure of its severity.
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease of hyperglycemia associated with metabolic syndrome, which is characterized by insulin resistance [1]. Long-standing DM affects many organs leading to severe complications of retinopathy, nephropathy, and neuropathy [2]. Plasma glycosylated hemoglobin (HbA1c) is as an index of average glycemic control over the previous 2–3 months and indicates poor diabetic control; furthermore, increased HbA1c concentration is the most important risk factor for predicting DM complications. Maintaining an HbA1c level below 6.5% is critical to decrease the incidence of diabetic complications [3]. Many investigators have examined the correlation of HbA1c levels with DM complications. However, very little research has focused on the critical HbA1c level in diabetic peripheral polyneuropathy.
Diabetic peripheral polyneuropathy, which includes peripheral nerve damage, is one of the most common complications of DM, affecting approximately 8% of newly diagnosed patients and >50% of patients with long-term DM [4]. It is defined as the presence of symptoms and/or signs of peripheral nerve dysfunction in DM patients after exclusion of other possible causes. The symptoms of polyneuropathy vary from painful sensory changes to motor weakness. Common symptoms are symmetrical paresthesia and burning pain that predominantly occurs distally in the legs according to length-dependency. Severe complications can result in foot ulceration and nontraumatic amputation [5]. Because no treatment for diabetic peripheral neuropathy is available, its prevention and early detection is of utmost importance. However, because not all sensory and motor symptoms in the extremities correspond to an electrophysiological abnormality, screening for diabetic peripheral polyneuropathy in DM patients poses a challenge. Therefore, a quantified, reliable screening tool is required for early detection of diabetic peripheral polyneuropathy.
Electrodiagnostic testing, including a nerve conduction study (NCS), has been the gold standard for diagnosing diabetic peripheral polyneuropathy with nerve dysfunctions. However, NCS is not easily applicable as a screening tool since it is uncomfortable for the patient and time-consuming [6]. In addition, standardization of the scoring system and accurate quantification of disease severity have not been fully established for diabetic peripheral polyneuropathy testing [7]. A few studies reported that the electrophysiological severity was correlated with poor diabetic control [8910]. However, these studies were limited by lack of statistical correction for the other risk variables, and thus, it could not be performed to analyze the independent effect of HbA1c level on the severity of diabetic peripheral polyneuropathy. Subsequently, a composite score has been introduced for quantitative analysis of the results of NCS [8]. It is considered a more valid evaluation tool for the severity of polyneuropathy than the single attribute of nerve conduction [11]. Furthermore, the HbA1c level could be a screening tool for early detection of diabetic peripheral polyneuropathy and an indicator of the optimal time for performing NCS in patients with long-standing diabetes. Moreover, the quantified composite score of NCS may be associated with disease severity.
The purpose of this study was to determine comparative risk factors for polyneuropathy in DM patients. The correlations of the risk factors with the quantified polyneuropathy severity were evaluated and the independent regression coefficients were compared.
MATERIALS AND METHODS
Subjects
A retrospective analysis was conducted on type 2 diabetic patients referred from the Department of Internal Medicine between March 2011 and September 2014 for evaluation for diabetic peripheral polyneuropathy. All patients had received treatment for more than 1 year and a Diabetic Neuropathy Symptom score [12] of at least 1. The scoring was composed of four-items: 1) unsteadiness in walking; 2) pain, burning, or aching in legs or feet; 3) prickling sensations in legs or feet; and 4) numbness in legs or feet. After performing electrodiagnostic testing, 153 diabetic patients without electrophysiological abnormality (DM-PN group) and 34 diabetic patients with polyneuropathy (DM+PN group) were selected. Patients with other concomitant diseases that could cause peripheral polyneuropathy, such as alcohol abuse, liver or renal disease; patients using anticancer or antituberculosis or antiarrhythmic agents; and patients with a previous brain or spinal cord injury or history of lumbar or cervical radiculopathy were excluded. Electrophysiologically, if the patient displayed focal entrapment neuropathy, the involved limb was excluded from the diagnostic decision of diabetic polyneuropathy, and in case of bilateral lesions, the patient was excluded from the study. This study was reviewed and approved by the local ethics committees.
Methods
Medical inspection
For patients, age, sex, height, weight, duration of DM, and plasma HbA1c level were obtained retrospectively through medical records. Hypertension and lipid profile, known to be associated with metabolic syndrome, were also checked [13]. The plasma levels of HbA1c and lipid profile at the time of conducting electrodiagnostic study were used in the analyses.
DM was diagnosed by an endocrinologist based on the criteria of fasting glucose >126 mg/dL or random glucose >200 mg/dL with polyuria and polydipsia [14]. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Blood samples for total cholesterol, triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were collected after a 10- to 12-hour overnight fast.
For comparing adjusted relative risks through binomial regression analysis, age, DM duration, HbA1c level, and lipid profiles were defined as follows: old age, ≥65 years [15]; chronicity of DM, duration of ≥10 years; increased HbA1c, ≥6.5%; hypercholesterolemia, ≥200 mg/dL; hypertriglyceridemia, ≥1,500 mg/dL; decreased HDL, <40 mg/dL; and increased LDL, ≥130 mg/dL [3].
Electrodiagnostic testing
Electrodiagnostic testing including the NCS was performed by rehabilitation physicians using a Keypoint EMG machine (Dantec, Copenhagen, Denmark). Recordings were performed with temperature control (32℃–34℃), careful distance measurements, and well-defined and artifact-free responses. NCSs were performed on peroneal, tibial, median, and ulnar nerves for the motor parameters; and sural, median, and ulnar nerves for the sensory parameters, according to the recommendation of the American Association of Neuromuscular & Electrodiagnostic Medicine [16]. The study was conducted on three extremities, and the polyneuropathy was diagnosed when abnormal parameters were present simultaneously in at least two limbs. The reference values for the NCS were shown in Table 1.
In the electrophysiological nerve study, the composite score was composed of 5 items: peroneal motor distal latency, peroneal motor amplitude, peroneal motor conduction velocity, tibialmotor distal latency, and sural sensory amplitude. Each item was scored as 0 at ≤95.0 percentile, 1 at 95.0–99.0 percentile, 2 at 99.0–99.9 percentile, and 3 at ≥99.9 percentile relative to the distribution of the values of normal NCS. The scores of the 5 items were summed, divided by the number of attributes with obtainable values, and then multiplied by 5 [8]. The reference points related with percentile value were as follows (95.0, 99.0, and 99.9 percentile): peroneal motor distal latency (4.8, 5.9, and 6.0 ms), peroneal motor amplitude (1.8, 0.6, and 0.5 mV), peroneal motor conduction velocity (41.7, 41.2, and 41.1 m/s), tibial motor distal latency (4.8, 4.9, and 5.0 ms), and sural sensory amplitude (6.2, 3.8, and 3.5 µV).
Statistical analysis
All statistical analyses were performed using SPSS for Windows ver. 17.0 (SPSS Inc., Chicago, IL, USA). For comparisons between the two groups, independent t-tests and χ2 tests were used for continuous variables and categorical variables, respectively. The variables with p-value<0.3 by univariate analysis were analyzed with binomial logistic regression analysis to estimate their independent effects on polyneuropathic involvement, and odds ratios and 95% confidence intervals were calculated for each variable. For the continuous variables, normality was tested according to Shapiro-Wilk test, and then multiple regression analysis was used, to assess the association with composite score. Null hypotheses of no difference were rejected for p-values<0.05.
RESULTS
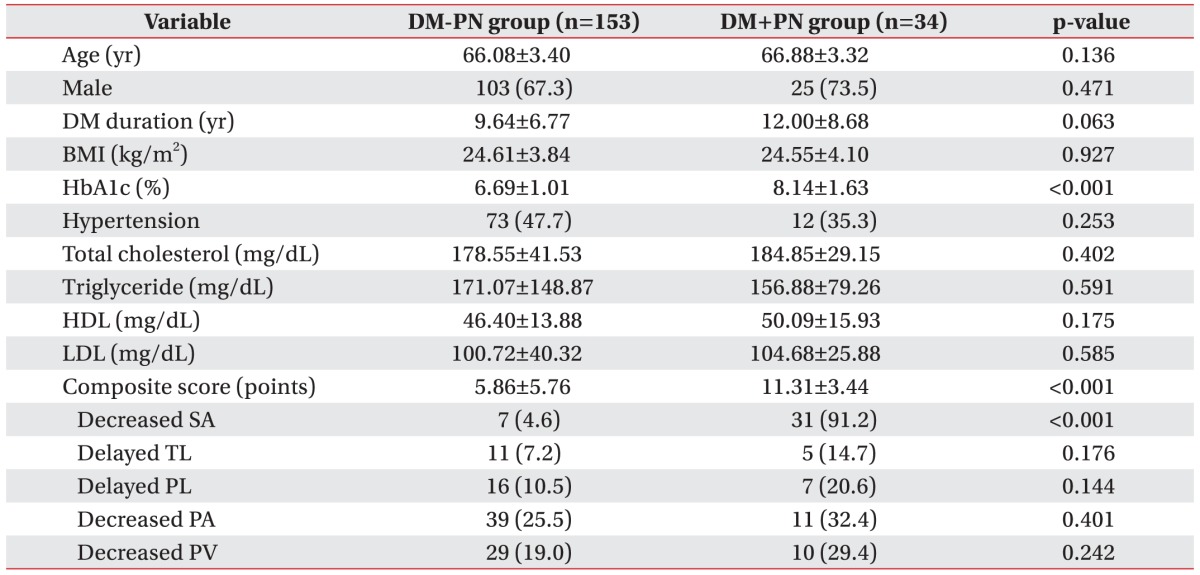
The comparative specific clinical and laboratory factors between the DM+PN and DM-PN groups were evaluated (Table 2). HbA1c level and composite score showed significant differences between the groups (p<0.001 for both). The DM+PN group showed higher HbA1c level and composite score than those of DM-PN. There were no statistically significant differences between the groups for other factors including age, male sex, DM duration, BMI, hypertension, total cholesterol, triglyceride, HDL, and LDL.
Among the 5 components of the composite score, 7 DM-PN patients (4.6%) and 31 DM+PN patients (91.2%) showed abnormal sural nerve amplitude, with statistically significant difference (p<0.001). The other components including delayed tibial latency, delayed peroneal latency, decreased peroneal amplitude, and decreased peroneal velocity were not significantly different between the groups.
In the univariate analysis for the categorical variables, there were significantly high proportions of old age (31/34, 91.2%) and increased HbA1c (28/34, 82.4%) in DM+PN patients (p=0.006 and p<0.001, respectively) (Table 3). The DM+PN group showed higher tendency to the ratio of chronic DM and hypertension, without statistical significance (p=0.183 and p=0.253, respectively). There were no significant differences for the male sex, obesity, hypercholesterolemia, hypertriglyceridemia, decrease HDL, and increased LDL between groups. Logistic regression analysis for age, DM duration, HbA1c level, and hypertension, indicated that age and HbA1c level were significant predictive factors for early detection of diabetic polyneuropathy. However, DM duration and hypertension were not independent, predictive factors for diabetic polyneuropathy.

Univariate and multivariate analyses for the categorical variables of risk factors related to polyneuropathy in diabetic patients
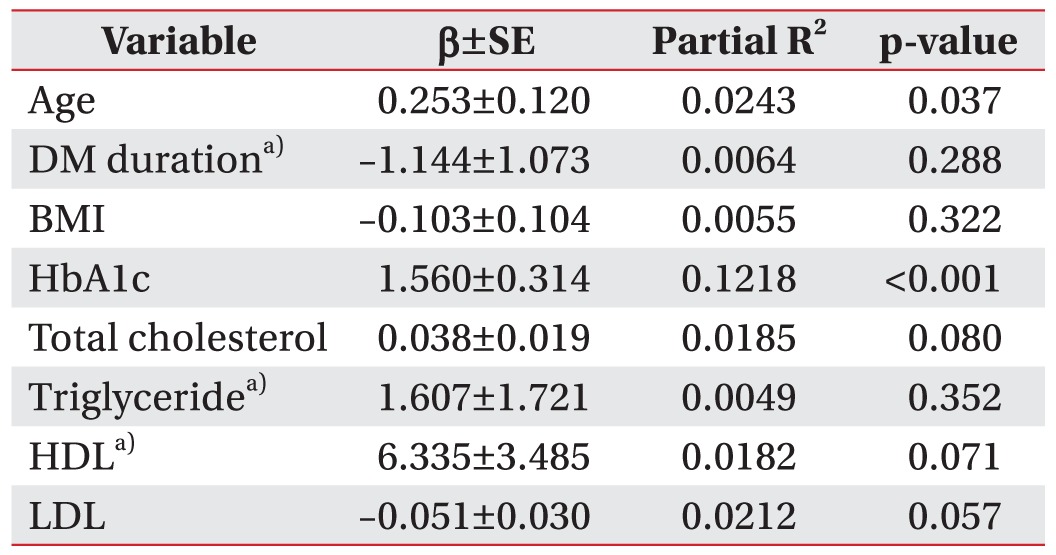
Furthermore, the correlation of individual risk factors with the composite score was determined for the entire cohort of diabetic subjects. Log-transformed values were used for DM duration, triglyceride, and HDL, because the continuous variables did not satisfy the normality assumption. In the multiple linear regression model (Table 4), HbA1c and age showed a significantly positive association with composite score, more strongly associated in HbA1c than age (β=1.560, p<0.001 and β=0.253, p=0.037, respectively); these differences were observed through a scatter plot (Fig. 1). However, other continuous variable of risk factors including DM duration, BMI, total cholesterol, triglyceride, HDL, and LDL were not significantly associated with composite score.
DISCUSSION
The purpose of the current study was to determine the significant risk factors for diabetic peripheral polyneuropathy and verify their value as a screening tool to diagnose diabetic polyneuropathy. Our results showed that HbA1c level was a quantitative indicator of the severity of polyneuropathy; and poor glycemic control (HbA1c level >6.5%) could increase the risk for the concurrence of polyneuropathy in DM patients by more than 5-fold.
Some studies have focused on associations between blood glucose control and diabetic peripheral polyneuropathy. However, majority of the studies had the limitation of analysis of deterioration in an individual nerve, since standardization for electrophysiological diagnostic criteria and quantification for multiplicity of the peripheral nervous system are still controversial [1718]. There is an ongoing need to quantify the severity of polyneuropathy; in fact, Dyck et al. [811] validated the composite score as a quantitative measure for polyneuropathy from a longitudinal study on a large cohort of 870 diabetic patients. The relationship between diabetic peripheral polyneuropathy severity and various physical parameters using this composite score have been reported [1019].
In the present study, age and HbA1c level were independent risk factors for comorbidity of polyneuropathy in DM patients. However, when the investigation was expanded from a binominal analysis confined to whether the patients had a polyneuropathy or not, quantified polyneuropathy severity of the composite score showed more significantly positive association for HbA1c level than age. The results indicated that the degree of hyperglycemia reflected the severity of polyneuropathy as well as increased the risk for the concurrence of polyneuropathy in DM patients by more than 5-fold. However, age was less associated specifically with the severity of polyneuropathy, consistent with previous studies reporting decreased peripheral nerve function in older diabetic patients [20], as well as in a nonspecific aged population [21]. When performing a NCS for the diagnosis of diabetic peripheral polyneuropathy, HbA1c level may be actively referred to as the index of polyneuropathy severity; whereas, age might be passively considered for the possibility of physiologic neural degeneration from the aging process itself. Considering the disadvantages of the electrophysiological test as time-consuming and causing patient discomfort [22], it may be helpful to check the glycemic control status, combined with NCS, for the evaluation of diabetic patients with clinically suspected polyneuropathy.
In this study, there were no significant differences in obesity, hypertension, hyperlipidemia, and duration of DM between diabetic patients with and without polyneuropathy. Unlike our findings, metabolic syndrome is reportedly correlated with type 2 DM [123], however, the risks and the disease-related algorithm of the individual components composing the syndrome remain controversial [24]. DM duration is a well-known risk factor for the chronic microvascular complications [10], however, intensive blood glucose control is also known to significantly reduce the diabetic complications than standard control for the same disease duration [25]. History taking depends mainly on the subjective state of the patient, hence, there can be a recall bias and discrepancy between the point of actual morbidity and the time of diagnosis. Therefore, in the medical examination of diabetic patients presenting with neuropathic symptoms, a careful inspection of detailed patients' history and chart review, as well as the status of glycemic control might be needed. Electrophysiologically, abnormality of sural sensory amplitude was the main contributor to the severity of diabetic peripheral polyneuropathy, which disagrees with an earlier study reporting that conduction velocity of the peroneal motor nerve was the main contributor; therefore, quantitative evaluation of the severity of diabetic peripheral polyneuropathy is important, together with a continued discussion of the overall quantification.
There are some limitations in the current study. First, it was difficult to apply corrected criteria on the various independent variables, including age and disease duration, due to the retrospective nature of the study. Second, comparison of serial electrophysiological changes was not conducted owing to a lack of follow-up evaluation; therefore, our cross-sectional findings need to be confirmed on further research. In the future, a large-scale, long-term prospective study with corrected variables comparing laboratory and electrodiagnostic testing would be necessary to investigate how the chronic glycemic control affects the prognosis of diabetic polyneuropathy, by inspecting the change of HbA1c level. Furthermore, the way of summing up the weighted values according to percentile might need verification, and universally valid criteria require further study, for the diagnostic standard of peripheral polyneuropathy.
In conclusion, our study demonstrated that an increased HbA1c level indicative of chronic hyperglycemia, could significantly increase the risk and quantitatively reflect the severity of polyneuropathy in diabetic patients.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.