Crystallization of Local Anesthetics When Mixed With Corticosteroid Solutions
Article information
Abstract
Objective
To evaluate at which pH level various local anesthetics precipitate, and to confirm which combination of corticosteroid and local anesthetic crystallizes.
Methods
Each of ropivacaine-HCl, bupivacaine-HCl, and lidocaine-HCl was mixed with 4 different concentrations of NaOH solutions. Also, each of the three local anesthetics was mixed with the same volume of 3 corticosteroid solutions (triamcinolone acetonide, dexamethasone sodium phosphate, and betamethasone sodium phosphate). Precipitation of the local anesthetics (or not) was observed, by the naked eye and by microscope. The pH of each solution and the size of the precipitated crystal were measured.
Results
Alkalinized with NaOH to a certain value of pH, local anesthetics precipitated (ropivacaine pH 6.9, bupivacaine pH 7.7, and lidocaine pH 12.9). Precipitation was observed as a cloudy appearance by the naked eye and as the aggregation of small particles (<10 µm) by microscope. The amount of particles and aggregation increased with increased pH. Mixed with betamethasone sodium phosphate, ropivacaine was precipitated in the form of numerous large crystals (>300 µm, pH 7.5). Ropivacaine with dexamethasone sodium phosphate also precipitated, but it was only observable by microscope (a few crystals of 10–100 µm, pH 7.0). Bupivacaine with betamethasone sodium phosphate formed precipitates of non-aggregated smaller particles (<10 µm, pH 7.7). Lidocaine mixed with corticosteroids did not precipitate.
Conclusion
Ropivacaine and bupivacaine can precipitate by alkalinization at a physiological pH, and therefore also produce crystals at a physiological pH when they are mixed with betamethasone sodium phosphate. Thus, the potential risk should be noted for their use in interventions, such as epidural steroid injections.
INTRODUCTION
The particle size and aggregation pattern of corticosteroids used in epidural injection are widely known to clinicians. Dexamethasone sodium phosphate does not contain particles or aggregate significantly. In contrast, triamcinolone acetonide has particles of 0.5–110 µm in size and develops evident aggregation [123]. In cases of an unintended intra-arterial injection of the particulate corticosteroid, the particles or aggregates much larger than blood cells can cause embolic brain or spinal cord infarction; clinicians who conduct spinal intervention are ever concerned about this issue [456].
However, the crystal formation of local anesthetics used in epidural injection has not been well studied. Local anesthetics are weak bases (e.g., lidocaine, bupivacaine, and ropivacaine have dissociation constants [pKa] of 7.7, 8.1, and 8.1, respectively), and most consist of an aromatic ring connected to an amine group with an amide bond, and have poor solubility in water [78]. Such properties suggest that they may precipitate in basic condition. Many commercial corticosteroid solutions include weak bases such as dexamethasone sodium phosphate and betamethasone sodium phosphate. As such, local anesthetics may flocculate when they are mixed with commercial corticosteroids. In our experience, precipitation was clearly observed in selective combinations of local anesthetic and corticosteroid (Fig. 1). Such flocculation of injectate represents a potential hazard, in vivo. Thus, in this study, we aimed to test at which pH level various local anesthetic solutions produce crystal, and to confirm which combination of corticosteroid and local anesthetic yields crystal.
MATERIALS AND METHODS
Materials
'Ropivacaine-HCl 0.75%' (Hanlim Pharm. Co. Ltd., Seoul, Korea), 'bupivacaine-HCl injection 0.5%' (Myungmoon Pharm. Co. Ltd., Bucheon, Korea), and 'lidocaine-HCl injection 1%' (Daihan Pharm. Co. Ltd., Seoul, Korea) were the local anesthetics used. To evaluate the crystal formation of the local anesthetics in mixture with corticosteroids, 'triamcinolone acetonide 40 mg/mL' (Shinpoong Pharm. Co. Ltd., Seoul, Korea), 'dexamethasone sodium phosphate 5 mg/mL' (Daewon Pharm. Co. Ltd., Seoul, Korea), and 'betamethasone sodium phosphate 5.2 mg/mL' (Daewon Pharm. Co. Ltd.) were used. To observe at which pH condition crystals of the local anesthetics are formed, sodium hydroxide (NaOH) solutions at 4 different concentrations (1:1, 1:10, 1:100, and 1:1,000 dilutions of 0.1 M NaOH solution) were mixed with the local anesthetics. Insoluble crystal formation was confirmed by light microscopy (CX40-12J02; Olympus, Tokyo, Japan). The pH of the solution was measured by pH/mV/temperature meter (XL15; Fisher Scientific, Pittsburgh, PA, USA).
Methods
The pH and particle size of each local anesthetic and corticosteroid
The default pH of 4 mL of each of the 3 commercial local anesthetics (ropivacaine-HCl, bupivacaine-HCl, and lidocaine-HCl) and 3 corticosteroids (triamcinolone acetonide, dexamethasone sodium phosphate, and betamethasone sodium phosphate) was measured using a pH meter. The existence of particles of the local anesthetic or corticosteroid was evaluated by light microscopy.
Precipitation of the local anesthetics in various pH conditions
Two milliliters of local anesthetic solution (ropivacaine-HCl, bupivacaine-HCl, or the lidocaine-HCl) was mixed with 2 mL of NaOH solution at 4 different concentrations (1:1, 1:10, 1:100, and 1:1,000 diluted NaOH solutions). The pH of each mixture was measured using a pH/mV/temperature meter. Irrespective of insoluble crystal formation, each mixed solution was observed with the light microscope and images were acquired. If crystals were observed on the microscope, the crystals were measured.
Insoluble crystal formation of local anesthetics in mixture with various corticosteroids
Two milliliters of local anesthetic solution (ropivacaine-HCl, bupivacaine-HCl, or the lidocaine-HCl) was mixed with 2 mL of corticosteroid solution (triamcinolone acetonide 80.0 mg, dexamethasone sodium phosphate 10.0 mg, or betamethasone sodium phosphate 10.4 mg solutions). pH measurement and the microscopic observation were conducted for each mixture. In case of crystal formation, the crystals were measured.
RESULTS
The pH and particle size of each local anesthetic and corticosteroid
We tested the pH of the 3 local anesthetics under examination; all of the local anesthetics-HCl solutions were weakly acidic. Ropivacaine solution was most acidic, pH 6.2, and bupivacaine solution followed at pH 6.4. The pH of the lidocaine solution was 6.7.
We also tested the pH of the corticosteroids under examination. Triamcinolone acetonide was the only acidic corticosteroid solution (pH 6.1). Betamethasone sodium phosphate was most basic (pH 8.8), and dexamethasone sodium phosphate was less basic (pH 7.7) (Table 1).
No identifiable particles were observed in unmixed solutions of dexamethasone, betamethasone, ropivacaine, bupivacaine, or lidocaine. Triamcinolone acetonide solution contained particles of several micrometers in size that formed aggregates of hundreds of micrometers in size.
Precipitation of local anesthetics in various pH conditions
Ropivacaine-HCl and bupivacaine-HCl showed no precipitation that could be seen by the naked eye or by microscope, when they were mixed with the 1:1,000 NaOH solution (Fig. 2A, E). The pH of the mixtures were 6.8 and 7.5, respectively. In the mixtures with 1:100, 1:10, or 1:1 NaOH solutions (pH 6.9, 12.1, and 12.8, respectively), ropivacaine-HCl produced insoluble particles and it was observed as a cloudy appearance by the naked eye. The particles were less than a few micrometers in size, but aggregations were found to be larger than hundreds of micrometers in size in the mixtures with 1:10 or 1:1 NaOH solution. The aggregation was larger in more basic conditions, so much so that the size of the aggregate ranged beyond 500 µm at pH 12.8 (Fig. 2B–D).
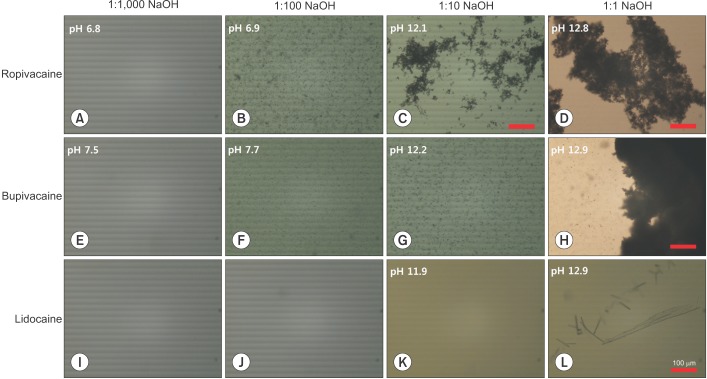
Microscopy imaging of precipitation of local anesthetics in variable pH conditions (100×); the scale bar indicates 100 µm. (A–D) Ropivacaine precipitated in solutions of pH 6.9 or higher. Higher precipitation and aggregation were observed at higher pH. (E–H) Bupivacaine began to produce insoluble particles at pH 7.7, and (I–L) lidocaine developed crystals at pH 12.9.
Similarly, bupivacaine-HCl produced insoluble particles in mixtures with 1:100, 1:10, or 1:1 NaOH solution (pH 7.7, 12.2, and 12.9, respectively), and it looked cloudy to the naked eye. The particles found were smaller than a few micrometers in size. In the mixture with 1:1 NaOH solution, however, aggregates were larger than hundreds of micrometers (Fig. 2F–H).
Lidocaine-HCl showed no precipitation when it was mixed with 1:10 NaOH solution or less basic solutions (i.e., when the pH was 11.9 or less in the mixture) (Fig. 2I–K). In the mixture with the 1:1 NaOH solution, lidocaine-HCl produced insoluble crystals (pH 12.9) (Fig. 2L). The crystal size was often seen to be larger than 400 µm, but was fragile. The precipitation was also visible to the naked eye, in the form of a cloudy appearance.
Insoluble crystal formation of local anesthetics in mixture with various corticosteroids
Any of the 3 tested local anesthetics (ropivacaine, bupivacaine, and lidocaine), when mixed with triamcinolone acetate, were weakly acidic (pH 6.1, 6.3, and 6.5, respectively). Additional crystals were not observed apart from the triamcinolone particle itself.
Ropivacaine-HCl mixed with dexamethasone sodium phosphate, was pH 7.0, and no precipitation was observed to the naked eye. However, a few rod-shaped crystals, 10–100 µm in size, were observed by microscopy. The mixture of ropivacaine-HCl and betamethasone sodium phosphate was weakly basic (pH 7.5). The solution looked cloudy to the naked eye, containing visible precipitations. With the microscope, this solution showed many more and larger (>300 µm) crystals than the mixed solution of ropivacaine and dexamethasone.
Bupivacaine-HCl rarely formed crystals when mixed with dexamethasone sodium phosphate (pH 7.3). It did however develop numerous round, insoluble particles, a few micrometers in size without obvious aggregation, in the mixture solution with betamethasone sodium phosphate (pH 7.7), appearing cloudy macroscopically.
Lidocaine-HCl did not precipitate in mixture with either dexamethasone or betamethasone (pH 7.2 and 7.7, respectively).
All microscopic analysis of mixed solutions of local anesthetics and corticosteroids are represented in Fig. 3.
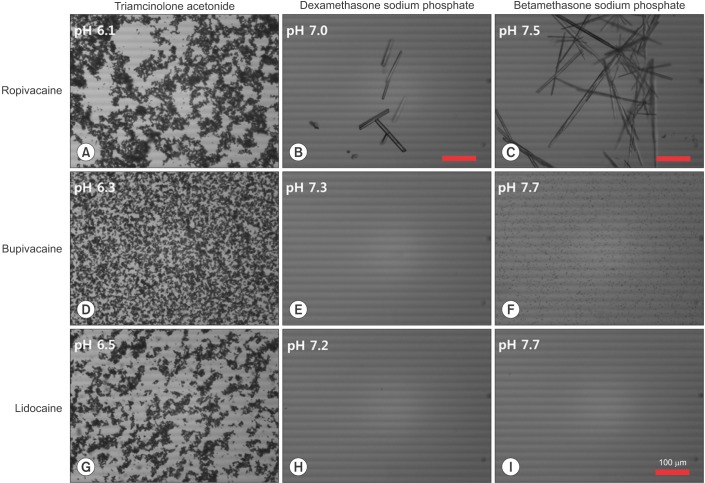
Microscopy imaging of local anesthetics mixed with corticosteroid solutions (100×); the scale bar indicates 100 µm. Ropivacaine developed crystals in the mixed solution with either dexamethasone sodium phosphate (B) or betamethasone sodium phosphate (C). Many more and larger crystals were created at higher pH in solution with betamethasone, compared to when in solution with dexamethasone (pH 7.5 and pH 7.0, respectively). Bupivacaine precipitated in solution with betamethasone sodium phosphate (F, pH 7.7), but not in solution with dexamethasone sodium phosphate (E, pH 7.3). Lidocaine did not create any precipitate (H, I). Triamcinolone acetate did not induce precipitation of any local anesthetics tested, but the particles of triamcinolone itself were observed (A, D, G).
DISCUSSION
The commercially used local anesthetics are maintained in acidic conditions (for example, ropivacaine-HCl pH 6.2, bupivacaine-HCl pH 6.4, and lidocaine-HCl pH 6.7). When the pH of the solution increased to a certain value (ropivacaine pH 6.9, bupivacaine pH 7.7, and lidocaine pH 12.9), precipitation of the local anesthetics occurred, and it could be observed with the naked eye or by microscope. This precipitation and aggregation increased with higher pH.
When ropivacaine-HCl was mixed with the same volume of basic corticosteroid solution—dexamethasone sodium phosphate (pH 7.7) and betamethasone sodium phosphate (pH 8.8), ropivacaine became alkalinized (pH 7.0 and 7.5, respectively), and precipitation was observed; a greater number of larger crystals were observed in the betamethasone mixture when compared to those formed in the dexamethasone mixture. Among the mixed solutions of bupivacaine with the same volume of corticosteroid, only betamethasone sodium phosphate solution induced observable precipitation (pH 7.7). No flocculation was observed in the solutions of lidocaine mixed with dexamethasone or betamethasone sodium phosphate.
These results are in close agreement with earlier studies that demonstrated ropivacaine and bupivacaine precipitation when they are alkalinized to physiologic pH with sodium bicarbonate, and lidocaine does not [9101112]. However, this phenomenon has not been emphasized in clinical journals or widely appreciated by physicians. Moreover, this is the first report that shows local anesthetics can precipitate when mixed with basic corticosteroid solutions that are widely used in clinical practice.
The mechanism by which this precipitation of local anesthetics occurs can be explained. Since the ratio of ionized to neutral base follows the Henderson-Hasselbalch equation (pH=pKa+log [unionized form]/[ionized form]), the proportion of the unionized base form of a local anesthetic increases with alkalinization. Precipitation increases with alkalinization because these unionized forms tends to be relatively insoluble in water [7].
In our microscopic observations, precipitation patterns of local anesthetics were different depending on the base used (NaOH vs. corticosteroid sodium phosphate). In mixtures with NaOH solution, large aggregates (>100 µm) of small particles (<10 µm) were formed. In contrast, large crystals (>100 µm) could be generated in the mixed solutions with corticosteroids. In the literature, local anesthetics are known to have crystal polymorphisms with different thermodynamic stabilities [13]. One reason for the different precipitation patterns is thought to be the difference in the reaction rate. The low initial pH difference between the local anesthetic and corticosteroid solution may mean that the reaction rate is (relatively speaking) slow enough to develop large crystals. The larger molecules of corticosteroids could also contribute to the crystal formation as roles of condensation nuclei.
The flocculation may reduce the bioavailability of local anesthetics in vivo, but additional adverse effects or influences on the efficacy of corticosteroids are not well known. The size of crystals or aggregations of local anesthetics can be more than 10 times the size of red blood cells in vitro. If the precipitate is not broken into smaller pieces within the blood vessels, it may cause embolization. However, in vivo conditions differ from in vitro, in terms of pH, buffers, and temperature. The clinical consequences of crystalized local anesthetics cannot be assumed based on the results of this study.
Nonetheless, precipitates of local anesthetics may sustain in vivo. The results of the current study were obtained at room temperature (25℃); when the mixed solutions containing crystals were warmed to 40℃, flocculation was not obviously changed (data not shown). This is in agreement with a previous study that examined the effect of temperature on precipitation of local anesthetics [14]. We also tested if the crystals could be dissolved with acidification by adding HCl. Precipitates were confirmed to break down in strong acid (pH 1.0), so it was inferred to be dissolved in a certain acidity. However, we observed that the flocculation of ropivacaine remained until pH 6.9. In our anecdotal experience, independent of the current study, crystalized ropivacaine mixed with betamethasone was maintained within the muscle tissue after the injection (Supplementary Fig. 1). However, we still cannot be sure whether the crystals would dissolve, or not, in blood. Further research is warranted to assess the clinical significance and possible dangers of the crystallization of local anesthetics.
From the results of this study, there are a few areas for consideration in clinical practice. During last few years, ropivacaine-HCl has been more widely used than bupivacaine-HCl because of its reduced neurotoxicity and cardiovascular toxicity [15]. However, ropivacaine may require more watchful concern in terms of precipitation in mixture with basic corticosteroid solutions. Likewise, prudence is needed in the use of the corticosteroid, betamethasone sodium phosphate, when combined with local anesthetics. Betamethasone sodium phosphate is being increasingly used instead of triamcinolone acetate because triamcinolone is no longer recommended for epidural injections. However, in our study betamethasone sodium phosphate showed the strongest propensity for precipitating local anesthetics. If physicians are concerned about the potential hazard of precipitation of local anesthetics mixed with corticosteroid solution, the risk can be circumvented by sequential injection of local anesthetics before (or after) corticosteroid or by injecting only corticosteroid without the local anesthetic.
In conclusion, bupivacaine, ropivacaine, and lidocaine produce flocculation by alkalinization; ropivacaine and bupivacaine can precipitate even in physiological pH. Furthermore, ropivacaine and bupivacaine also produce crystals when they are mixed with basic corticosteroid solutions, particularly with betamethasone sodium phosphate. These potential risks should be noted prior to musculoskeletal interventions, such as epidural steroid injection, using corticosteroids and local anesthetics.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via http://dx.doi.org/10.5535/arm.2016.40.1.21. Fig. S1. The ultrasonic images of an occipital nerve block using betamethasone sodium phosphate and ropivacaine-HCl (A, before injection; B, after injection; white arrows, the needle). Crystallized ropivacaine remained in the muscle tissue without dissolution after the injection (white arrow heads).
Fig. S1
The ultrasonic images of an occipital nerve block using betamethasone sodium phosphate and ropivacaine-HCl (A, before injection; B, after injection; white arrows, the needle). Crystallized ropivacaine remained in the muscle tissue without dissolution after the injection (white arrow heads).