Percutaneous Adhesiolysis Versus Transforaminal Epidural Steroid Injection for the Treatment of Chronic Radicular Pain Caused by Lumbar Foraminal Spinal Stenosis: A Retrospective Comparative Study
Article information
Abstract
Objective
To investigate the efficacy of percutaneous adhesiolysis (PA) compared to fluoroscopy (FL)-guided transforaminal epidural steroid injection (TFESI) in patients with radicular pain caused by lumbar foraminal spinal stenosis (LFSS) by assessing pain relief and functional improvement at 4 and 12 weeks post-procedure.
Methods
This retrospective study included 45 patients who underwent PA or FL-guided TFSEI for radicular pain caused by LFSS of at least 3 months' duration. Outcomes were assessed with the Oswestry Disability Index (ODI) and Verbal Numeric Pain Scale (VNS) before the procedure and at 4 and 12 weeks post-procedure. A successful outcome was defined by >50% improvement in the VNS score and >40% improvement in the ODI score.
Results
ODI and VNS scores improved 4 and 12 weeks post-procedure in both groups. Statistically significant differences between groups were observed in ODI and VNS at 12 weeks (p<0.05). The proportion of patients with successful outcomes was significantly different between the two groups only at the 12-week time point.
Conclusion
Our study suggests that PA is effective for pain reduction and functional improvement in patients with chronic radicular pain caused by LFSS. Therefore, PA can be considered for patients with previous ineffective responses to conservative treatment. Although PA seems to be more effective than TFEFI according to the results of our study, in order to fully elucidate the difference in effectiveness, a prospective study with a larger sample size is necessary.
INTRODUCTION
Lumbar foraminal spinal stenosis (LFSS) is a common cause of radicular pain [1]. An 8% to 11% incidence of lateral root entrapment has been reported [123]. Although few studies have examined the mechanical compression of nerve roots in LFSS, it has been hypothesized that continuous compression of nerve roots damages microvessels and leads to ischemia, edema, demyelination, and C-fiber activation [4]. Various types of conservative treatments have been used for patients with radicular pain caused by LFSS. Fluoroscopy (FL)-guided transforaminal epidural steroid injection (TFESI) is considered the most precise and effective route of steroid administration [56]. The transforaminal approach is advantageous because corticosteroid preparations can be injected close to the probable source of the irritated nerve root, and this approach results in better ventral epidural spreading than does the interlaminar approach [56].
Although previous studies demonstrate efficacy of TFESI in managing lumbar radicular pain [678], epidural steroid injection is occasionally not effective in managing leg pain [8910]. It is thought that epidural fibrosis leads to imprecise drug delivery in some cases, which limits the effectiveness of TFSEI. Furthermore, epidural fibrosis effectively tethers the dura and nerve roots, causing a significant subset of patients to experience chronic radicular pain [111213]. The percutaneous adhesiolysis (PA) technique could overcome the limitations of TFESI. A PA catheter enters the ventral epidural space directly, advancing along the ventral epidural space (lateral recess to foramen) and directly breaking up perineural/epidural adhesions, fibrosis, or other resistive areas that are physical barriers to penetration of perineurally deposited drugs; this may enhance the delivery of high concentrations of injected drugs to the target area [111213]. A small number of studies have addressed the effectiveness of PA in patients with radicular pain caused by LFSS; also, only a few studies have compared the effectiveness and safety of PA and TFESI. Hence, the purpose of this retrospective study was to assess the relative effectiveness of PA and TFESI for managing radicular pain in patients with LFSS.
MATERIALS AND METHODS
Study design
We retrospectively compared data from patient charts. Patient privacy and data confidentiality were maintained throughout the research process. Approval from the Institutional Review Board of the Sanggye Paik Hospital was obtained. The approval included a waiver of informed consent, since the study did not include direct contact with the study population and all patient identifiers were removed from the data set upon initial collection.
Subjects
We studied data from patients with lumbar radicular pain caused by LFSS who visited our pain clinic between June 1, 2013 and June 31, 2014. Diagnoses were based on clinical profiles, physical examinations, electromyography tests, and computed tomography (CT) or magnetic resonance imaging (MRI) scans [14].
Those who met the following inclusion criteria were selected: aged 18 or older, received PA or FL-guided TFESI, no previous lumbar surgery, no central canal stenosis, no lateral recess stenosis no spondylolisthesis evident on MRI or CT, absence of progressive motor deficit or significant sensory deficit, and absence of cauda equina syndrome. We only included patients who had experienced chronic radicular pain for at least 3 months and had failed to respond to anti-inflammatory medications, analgesic therapy, or physical therapy for at least 4 weeks. Exclusion criteria included a diagnosis of sacroiliac joint or facet joint pain based on clinical or radiological evaluation, presence of definite motor weakness or hypoesthesia, presence of psychiatric disorders, and laboratory results suggesting bleeding disorders, infection, inflammatory disease, or rheumatoid disorders.
Percutaneous adhesiolysis
PA and FL-guided TFESI were both commonly used in our clinic to treat lumbar radicular pain caused by LFSS. Patients who failed to respond to more conservative management were informed of the potential risks associated with the two procedures and the benefits and risks of using corticosteroid mixed with contrast media, then asked to provide consent. The choice between PA and FL was made by the patient.
FL-guided TFESI was conducted by a physician (Y. Park) with >7 years of experience and PA was performed by a pain specialist (W. Y. Lee) with >3 years of experience. FL-guided TFESI was performed as an outpatient procedure and PA was performed in an operating room.
Before the PA procedure, an intravenous catheter was placed and antibiotics were administered. PA was performed using an EpiStim catheter (Sewoon Medical, Seoul, Korea) under sterile conditions using FL with continuous monitoring of vital signs (blood pressure, pulse, and heart rate). With the patient in the prone position, the site of needle insertion was sterilized with povidone iodine and draped. The FL was adjusted over the lumbosacral area such that a caudal approach could be used in both the anteroposterior and lateral views. After appropriate positioning of the FL, the precise needle insertion area around the sacral hiatus was determined and infiltrated with local anesthetics. A 15-gauge RK needle (Sewoon Medical) was inserted into the epidural space and a 19-gauge EpiStim catheter was advanced through the needle up to the 3rd sacral vertebra. An epidurogram was obtained after injecting approximately 2-5 mL of contrast media to confirm that the needle was placed in the epidural space and to avoid intravascular or subarachnoid needle placement. The tip of the catheter was positioned to address the adhesion site as identified by filling defects and suspected pain source. Subsequently, adhesiolysis and decompression were carried out by distension with a mixture 10 mL of 0.25% bupivacaine and 3% hypertonic saline, and by mechanical means using the catheter. When the catheter was placed in an area suspected to be the source of pain, some patients indicated that they felt pain similar to what they had been experiencing. After adhesiolysis, sufficient filling of the target nerve roots and epidural space was confirmed without intravascular, subarachnoid, or extra epidural injections. Then, a mixture of 5 mL 0.25% bupivacaine, 2 mg beta-methasone, and 1,500 units of hyaluronidase was slowly injected (Fig. 1A). After completion of the procedure, the patient was placed in a supine position and transferred to the recovery room. In the recovery room, the patient was closely monitored for any potential complications or adverse effects before being discharged.
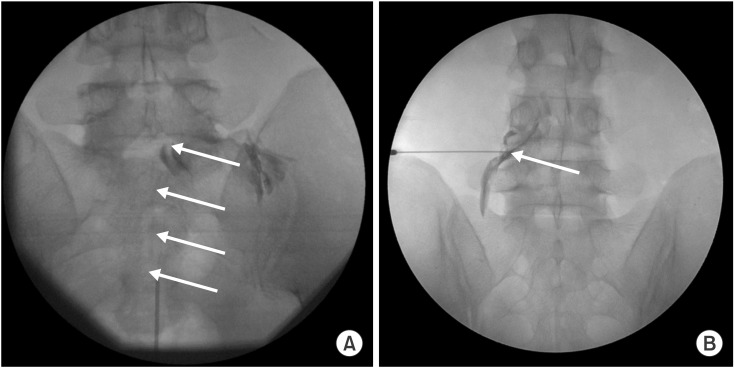
(A) Percutaneous adhesiolysis approach to the L5 nerve root. The anterior-posterior view shows the proper location of catheter in the L5-S1 foraminal canal (arrow). After adhesiolysis, sufficient filling of the target nerve roots and epidural space was confirmed without intravascular, subarachnoid, or extra epidural injections. Conventional transforaminal approach to the L5 nerve root. (B) Conventional transforaminal approach to the L4 nerve root. The anterior-posterior view shows the proper location of the needle at the base of the pedicle (arrow). A small amount of contrast media was used to confirm epidural spread.
Fluoroscopy-guided transforaminal epidural steroid injection
All patients were placed in a prone position with a pillow under the lower abdomen and above the iliac crest to reduce lumbar lordosis. The FL was adjusted so that an X-ray passed perpendicular to the endplate of the lower portion of the upper vertebrae, which is one of the two targeted spinal segments. After confirming overlap between the pedicle of the upper vertebrae and the superior articular process of the lower vertebrae from an oblique view, a spot directly inferior to the pedicle was identified as the target. The area was prepared for injection by applying an aseptic dressing after sterilizing the skin with betadine and alcohol. The tip of a 22-gauge, 3.5-inch spinal needle was gradually advanced from the 6 o'clock direction toward the spot directly inferior to the pedicle using radiologic guidance. Although the needle tip did not have to proceed all the way to the periosteum bone, we ensured that the tip was placed directly inferior to the pedicle and in the inferolateral area of the intra-articular space (i.e., safe triangle) via several radiologic views. When the tip touched the inferolateral border, the FL was turned to the lateral view, and the needle was advanced gradually toward the foramen in an anterior and superior direction. Drug delivery into the anterior epidural space was confirmed via injection of 1-2 mL radio contrast media under intermittent fluoroscopic imaging (Fig. 1B). If it was not observed, 2 mL of the drug (0.5% lidocaine 1.5 mL + triamcinolone 20 mg) was injected. We defined failure of TFESI as a 50% or less reduction on the Verbal Numeric Pain Scale (VNS) compared to pre-injection scores, when at least 2 injections were administered over 3 months.
Review of clinical data
Ninety-two patients (PA, n=28; FL-TFESI, n=64) received treatment during the time period encompassed by this study, 78 of whom (85%) met the inclusion criteria. Twenty-two (24%) patients were excluded because the patient did not complete and return the follow-up survey. Eleven (12%) patients were excluded because of the exclusion criteria listed above. Finally, 15 patients treated with PA and 30 patients treated with FL-TFESI were left. A standardized chart abstraction form was used to extract the collected data on demographics, treatment, pain severity, and functional assessment. Follow-up interviews were performed by nursing personnel not involved in the original procedure. The VNS and ODI were used to evaluate clinical effectiveness in terms of pain reduction and functional improvement at 4 and 12 weeks after the last injection. On the VNS, a score of 0 indicates no pain, and a score of 10 indicates the worst pain imaginable [15]. The ODI is one of the most commonly used disease-specific readouts for patients with LBP [16]. The ODI is consists of ten items, each of which is scored from 0 to 5; the total is added and multiplied by 2 to produce a final score in the range of 0-100.
Successful outcome was defined as a reduction in the VNS score of 50% or more, and successful functional improvement was defined as a reduction in the ODI of 40% or more at 4 weeks and 12 weeks after the procedure [17]. If the outcomes were not successful at 4 weeks, the patients were excluded at 12 weeks evaluation.
We reviewed patient charts to check for immediate adverse events (within a few minutes after the injection). Each patient was handed a questionnaire at the end of the procedure, and asked to complete it within 48 hours and return it at the 2-week follow-up visit.
Statistics
The chi-square test, Fisher exact test, and Mann-Whitney U test were used to compare the characteristics of the two groups in variables such as sex, age, body mass index (BMI), target nerve root, and pain duration. At each time point, VNS and ODI were compared by repeated measure analysis of variance (ANOVA), and Bonferroni correction was conducted for post-hoc comparison. The chi-square test was used to assess differences in proportions. Fisher exact test was used wherever the expected value was less than 5. Statistics were performed with SAS Enterprise Guide 4.1 (4.1.0.471); p<0.05 was considered statistically significant.
RESULTS
The average age of the patients was not significantly different between the two groups (PA, 61.1±12.8 years; FL-TFESI, 57.7±10.1 years). Significant differences were not observed in general characteristics of such as sex, age, BMI, target nerve root, disease duration, and imaging results (Table 1).
The ODI and VNS showed significant improvements 4 and 12 weeks post-procedure in both groups. A significant between-group difference was observed in ODI and VNS scores at 12 weeks post-procedure (Table 2). The proportion of patients with >50% improvement in VNS score and >40% improvement in ODI score is illustrated in Fig. 2, which shows a 73.3% improvement in the PA group and a 43.3% improvement in the FL group at 12 weeks. Successful treatments intend to be different between the groups at 12-week outcomes (p=0.057).

Illustration of significant pain relief (≥50% reduction in Verbal Numerical Pain Scale from baseline), functional improvement (≥40% improvement in the Oswestry Disability Index from baseline). A group is percutaneous adhesiolysis, B group is fluoroscopy-guided transforaminal epidural steroid. *p<0.05, significant difference from baseline values.
There were no cases of bent needle tips, torn catheters, sheared catheter remnants, or intrathecal placement of catheters. No instances of infection or hematoma were recorded. The only immediate adverse event reported was transient nerve irritation during the procedure, the incidence of which did not much differ between groups (FL-TFESI, n=4; PA, n=3).
DISCUSSION
The initial treatment of radicular pain associated with LFSS is conservative and includes mobilization exercises, anti-inflammatory medications, activity modifications, and epidural corticosteroid injections. TFESI may provide pain relief and functional improvement in cases of central or lateral recess stenosis, but often does not alter the symptoms of foraminal stenosis [18]. Although surgery may be recommended for patients who do not respond to conservative treatments, older individuals with comorbidities are not always good surgical candidates. In LFSS, narrowing of the bony foramen causes mechanical compression of spinal nerve roots. This dynamic mechanical compression of the nerve root sheath manifests as neural hyperemia, venous congestion, and edema. Venous congestion has been suggested as the essential factor precipitating circulatory disturbance, thus inducing neurogenic claudication [19]. Perineural fibrosis is closely related to venous obstruction and may further impede nutrient transfer and predispose to nerve stretch injury [20]. Also, fibrosis may extend into the neural canal and adhere to the dura mater and nerve roots, causing adhesion of nerve roots or dura, which may in turn contribute to persistent radicular pain and prevent delivery of medications for pain relief [21].
PA removes barriers in the epidural space thought to contribute to painful processes and prevent delivery of pain-relieving drugs [111221]. PA may also wash out inflammatory cytokines from the affected area [22]. A systemic review examined the relationship between the volume of fluid introduced during epidural corticosteroid injection and relief of radicular pain [23]. The study found that larger injection volume was associated with a greater magnitude of pain relief. The proposed mechanisms through which additional volume contributes to pain relief include not only adhesiolysis and washout of inflammatory cytokines, but also lavage of the epidural space, suppression of ectopic discharge from injured nerves, and enhanced blood flow to ischemic nerve roots [2223]. Previous studies investigated the efficacy of PA in lumbar spinal stenosis (LSS) [242526]. Manchikanti et al. [24] reported that 76% of patients who underwent PA obtained significant pain relief at a 12-month follow-up, whereas only 12% of those with caudal block obtained pain relief. Another study by Manchikanti et al. [25] showed that 89% of patients with moderate or severe LSS had significant pain relief at 3 months, but in that study they performed repetitive procedures. In a different retrospective study, of 63 patients who were followed up at 3 months, 34 (54.0%), 32 (50.8%), and 30 (47.6%) patients showed successful results on Numeric Rating Scale for back pain, Numeric Rating Scale for leg pain, and the ODI scale at 3 months, respectively [26].
In both studies by Manchikanti et al. [2526], repetitive procedures were done and foraminal stenosis patients were excluded; also, they one of those studies did not compare the effectiveness of PA to that of TFESI. To our knowledge, this is the first study comparing the effectiveness of TFESI and PA in patients with LFSS. The PA group showed significantly better therapeutic outcomes as measured by VNS and ODI at the 3-month follow-up.
In two retrospective studies evaluating the effectiveness of PA, poor outcomes were more common in patients with foraminal stenosis [2627]. Poor outcomes were attributable to the association of LSS with irreversible changes such as epidural scars and hypertrophy of bony structures and ligaments; such changes may render the nerve root refractory to management by the local application of corticosteroids [2829]. Such changes may also interfere with advancement of a catheter or injection of medication into the ventral epidural space and thus contribute to relatively poor PA outcomes [26]. Unlike in the studies mentioned above, our study showed a very successful therapeutic rate (70% success) in foraminal stenosis patients 3 months after the procedure. It should be noted that, first, the degree of severity in radiologic findings of LSS is known to not be proportional to symptom severity [3031]. Park et al. [32] demonstrated in cases of clinical LSS there was no association between PA outcome and anatomical degree of stenosis. In addition, Choi et al. [33] reported that LSS location was not a significant factor in the prognosis of PA. Secondly, the absence of previous lumbar surgery and root compression were identified to be good prognostic predictors of the results of PA [33]. Our study may have shown favorable outcomes since we included patients with root compression confirmed by radiologic examinations and excluded patients who had recently undergone surgery. Third, several studies have sought to determine whether epidural administration of hyaluronidase or hypertonic saline improves outcomes [22343536]. Yousef et al. [34] compared treatment outcomes in 38 subjects who received either FL-guided caudal injections of 10 mL 0.25% bupivacaine + 30 mL 3% hypertonic saline + 80 mg methylprednisolone, or the same mixture with 1,500 units of hyaluronidase added. In this small prospective study, only those patients who received hyaluronidase continued to experience benefits at 6 and 12 months post-procedure [34]. Two randomized studies conducted by the same group of investigators in patients with pain from failed back surgeries or sciatica compared high-volume interlaminar epidural injections of 5 mL 0.25% bupivacaine and 80 mg of triamcinolone, 5 mL 0.25% bupivacaine and 1,500 units of hyaluronidase, or a combination of bupivacaine, steroids and hyaluronidase [3536]. In both studies, greater improvement was noted in the group that received hyaluronidase and steroids than in those who received either drug alone. Thus, there is moderate evidence supporting the use of hypertonic saline in PA, and some evidence in favor of using hyaluronidase. Our study used hyaluronidase and hypertonic saline as well, which seems to have contributed to positive outcomes. Fourth, a significant portion of the benefit of PA can be attributed to high injection volume. In a systemic review, Rabinovitch et al. [23] found a strong correlation between the volume of fluid injected and pain relief irrespective of steroid dose in the immediate and intermediate term and a trend towards significance in the short term.
As is the case with any procedural intervention, bleeding, infection, and nerve damage are some of the general complications associated with PA. Previous, larger studies examined complications more extensively than we did. One retrospective review revealed a variety of different complications such as bent needle tips (4.8%), torn catheters during withdrawal (1.2%), sheared catheter remnants (0.4%), intrathecal placement of catheters (4.4%), and epidural abscesses (1.2%) [37]. In another large study, a prospective evaluation of 10,000 epidural injections found that of 839 patients who underwent adhesiolysis, the rates of intravascular injection (11.6%), transient nerve irritation (1.9%), and dural puncture (1.8%) were significantly higher than in patients who underwent conventional ESI [38]. No such fatal complication was reported in this study, and only transient nerve irritation was found during both TFESI (n=4) and PA (n=3).
The present study has several limitations. First, this study was retrospective in design. Although we selected subjects using valid inclusion and exclusion criteria, it is important to follow up our study with one that includes a more heterogeneous group of subjects. In addition, we could not evaluate the benefit of other treatments such as medication and physical therapy during the follow-up period. It is expected, however, that these treatments had no or minimal effects on the results because patients refractory to these treatments were chosen for this study. Second, the population was small. Even so, this study can be regarded as a significant first step that should lead to further inquiry on the effectiveness of TFESI and PA among foraminal stenosis patients. Third, a 3-month follow-up period is short. However, because the procedure was not repeated during the follow-up period, our results reflect the clinical efficacy of a single treatment and exclude the influences of repetition and the cumulative effects of multiple procedures. Fourth, the injected materials are different between the two groups. The TFESI group was injected with 0.5% lidocaine 1.5 mL + triamcinolone 20 mg, while the PA group was injected with 10 mL 0.25% bupivacaine + 3% hypertonic saline. Since there are differences in drug type as well as injection amount between the groups, this could be considered a method bias. Fifth, chronic lumbar foraminal stenosis is commonly accompanied by epidural adhesion. However, lumbar foraminal stenosis without adhesion is also found. Adhesions in the PA group were confirmed by filling defects, but no such assessment was done in the TFESI group. So if main pathologic condition is adhesion, we conclude that PA is the better treatment option, but if not, we cannot conclude that PA is more effective than TFESI in the treatment of chronic lumbar foraminal stenosis.
Our study suggests that PA is effective for pain reduction and functional improvement in patients with chronic radicular pain caused by LFSS. Therefore, PA should be considered for patients with previous ineffective responses to conservative treatment. Although PA seems to be more effective than TFEFI according to the results of our study, in order to make a more meaningful conclusion, a prospective study with a larger sample size will be necessary.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.

