Limb Differences in the Therapeutic Effects of Complex Decongestive Therapy on Edema, Quality of Life, and Satisfaction in Lymphedema Patients
Article information
Abstract
Objective
To investigate the changing patterns of edema, quality of life (QOL), and patient-satisfaction after complex decongestive therapy (CDT) in three trajectories: arm lymphedema (AL), secondary leg lymphedema (LL) and primary leg lymphedema (PL).
Methods
Candidates for AL (n=35), LL (n=35) and PL (n=14) were identified from prospective databases. The patients were treated with CDT for 2 weeks, and lymphedema volume was measured before and immediately following the therapy. Patients then self-administered home therapy for 3 months and presented for a follow-up visit. The Korean version of Short Form-36 (SF-36) was used to assess QOL, and we administered a study-specific satisfaction survey.
Results
There was no significant difference in the volume reductions between the 3 groups. There were no significant differences in all of the measures between PL and LL. Overall initial QOL was significantly lower in patients with LL than in patients with AL. SF-36 scores post-CDT did not differ significantly between AL and LL. Clinically significant differences were noted between AL and LL in the mean values of the satisfaction survey.
Conclusion
AL, LL, and PL may have different longitudinal courses. We suggest that lower extremity lymphedema patients present more favorable outcomes after CDT with respect to QOL and satisfaction than upper extremity lymphedema patients. Clinicians should approach patients with different therapeutic considerations specific to each type or region of lymphedema before using CDT in clinical practice.
INTRODUCTION
Lymphedema is a chronic disease that is associated with decreased physical, psychological, and social well-being as well as quality of life (QOL) [123]. The perception of the state of health and QOL of patients are widely recognized as important issues, and the treatment goals of patients with lymphedema include preservation of QOL [456].
Unfortunately, many of the treatment strategies used have not been proven to be successful in preventing the occurrence of lymphedema. Although secondary treatment is the best strategy for lymphedema, there is no definite treatment guideline [78910]. However, complex decongestive therapy (CDT) is currently recognized as a standard lymphedema treatment, focusing on reduction in limb volume and maintenance of healthy skin [91112]. CDT results in minimal loss of function and QOL [181314]. Interest has been stimulated by research into the effects of CDT on volume reduction and QOL [245151617].
In our experience, there are no distinct differences in the effectiveness of CDT in treating the two recognized types of lymphedema (primary and secondary). However, we have found that the clinical effectiveness of and satisfaction with CDT do not appear to be the same in patients with arm lymphedema (AL) and leg lymphedema (LL). Few studies have specifically compared the influence of CDT on QOL between limb groups and lymphedema types [101517], and well-designed studies are lacking. Moreover, there is little information about the reported efficacy of and satisfaction with CDT. In the clinical setting, the consequences of low treatment satisfaction with clinical treatment are a particular concern in patients with functional limitations. Therefore, understanding treatment effectiveness and the patient's view of CDT may help in the assessment of lymphedema patients.
The specific objective of this prospective study was to characterize the changing patterns of edema, QOL, and patient-satisfaction after CDT according to limb groups as well as lymphedema types.
MATERIALS AND METHODS
Subjects
This prospective study included patients with minimal to moderate, unilateral AL or LL between September 1, 2011 and August 31, 2012 attending an outpatient clinic at Samsung Medical Center. Approval for the study was obtained from the Ethics Committee, and written consent was received from all patients.
A physician diagnosed lymphedema on a clinical basis in patients who had developed a swollen limb. In patients with primary lymphedema, lymphatic scintigraphy was used to confirm the diagnosis. The inclusion criteria for this study were 1) unilateral lymphedema and 2) minimal to moderate lymphedema. The severity of lymphedema was determined using the International Society of Lymphology (ISL) criteria, which are based on volume differences and assessed as minimal (<20% increase), moderate (20%-40% increase), or severe (>40% increase) differences [7].
Because a normal contralateral limb was required to predict the unaffected limb size, patients with bilateral lymphedema were excluded. Patients with untreated or unstable medical conditions, current vascular disease, or a history of attempted lymphedema reduction in the affected limb, were also excluded.
Complex decongestive therapy
All patients received 10 CDT sessions of 90 minutes each, which included all of following components: manual lymph drainage massage, multilayered inelastic compression bandaging, and meticulous skin care [671118]. This intervention continued for 2 weeks (5 working days per week). For the first 60 minutes of each 90-minute session, manual lymphatic drainage was performed by certified therapists, followed by wrapping of the limb with short-stretch compression bandages and use of a sequential pneumatic pump for 30 minutes. During the 2 weeks of CDT, the patients were educated regarding the proper bandaging technique and medical remedial exercise to promote lymph drainage, maintenance of ideal body weight, and essentials of skin care during a comprehensive seminar. The patients were asked to massage daily, perform exercises, and care for their skin for the duration of the trial. Patients were fitted for compression garments (e.g., a stocking) after the initial 2 weeks of CDT. Clinical characteristics were obtained from a chart review on the day of CDT initiation.
Participants' enrollment and allocation
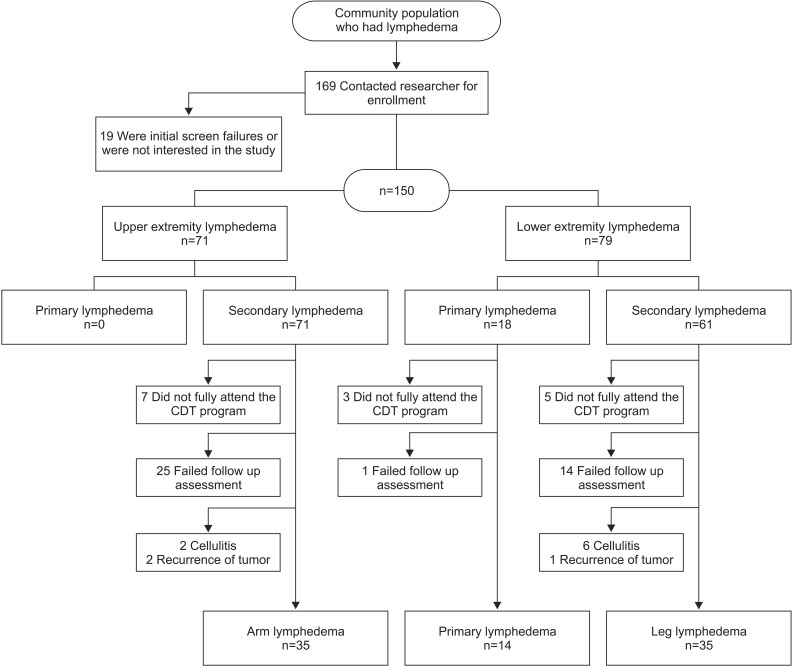
After consent, 150 patients of the eligible 169 patients with lymphedema underwent the pre-CDT volume check and independently responded to the QOL survey. Of these participants, 84 completed the 12-week visit and were included in the analysis. Of the 150 lymphedema patients, 15 did not fully attend the CDT program, 16 did not complete the post-treatment assessment, and 24 did not complete the 12-week assessment. Eleven patients were excluded because of an unstable medical condition or vascular disease during the follow-up period: 8 patients developed cellulitis, and 3 patients experienced recurrent tumors during the study (Fig. 1).
Outcome measurements
Limb volume measurements
Limb lymphedema volumes were measured using the electronic volumeter device (Perometer; Pero-System, Wuppertal, Germany), which is an optoelectronic instrument for measuring limb volume and circumferences [1920]. The measurements were obtained twice at each exam by another examiner, and these automatically calculated values (mL) were then averaged. Reproducibility of the Perometer has been confirmed by our institutions and by others [11181920].
The volume measurements were carried out 3 times in both groups: pre-CDT (day of treatment initiation), post-CDT (2 weeks after treatment initiation), and 12 weeks after the end of the treatments (14 weeks after treatment initiation). Patients who did not undergo all 3 measurements were excluded from analysis.
The severity of swelling was determined by subtracting the volume of the patient's unaffected limb from that of the affected edematous limb. The therapeutic response of CDT was quantified as the change in the percentage of excess limb volume (PCEV), which is the most common indicator of edema volume and calculated using the following formula [515182122]:
QOL questionnaire
QOL and health status were measured using the validated, self-administered, generic Short Form-36 (SF-36) health survey, which consists of 36 questions within 8 scales to assess health and functional status: physical function (PF), role limitation-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social function (SF), role limitation-emotion (RE), and mental health (MH) [2324]. The response to each question is rated from 0 (worst) to 100 (best), with higher scores indicating a better health status. The SF-36 has been previously and successfully used for patients with lymphedema [451617252627]. In the present study, the Korean version of the SF-36 was constructed for self-administration with each enrolled patient before each volume measurement [24].
Satisfaction questionnaire
After the CDT, a self-reported, study-specific survey reregarding the effectiveness of and satisfaction with the CDT was conducted. It consisted of 4 questions based on a 6-point Likert scale (0, extremely dissatisfied; 1, very dissatisfied; 2, somewhat dissatisfied; 3, somewhat satisfied; 4, very satisfied; and 5, extremely satisfied) (Appendix A). The questionnaire also included demographic items and questions associated with the details of the lymphedema. When requested, a trained physical therapist assisted the patients with the questionnaire.
Statistical analysis
The baseline demographic profiles were compared between two conditions in two groups (AL and LL, and LL and primary leg lymphedema [PL]) using independent t-tests. The primary outcomes of SF-36 score and reduction in PCEV were compared from baseline to 2 weeks and 14 weeks using repeated measured ANOVAs, independent t-tests and paired t-tests. These analyses were conducted with limbs or causes as the primary factor, and time (pre-CDT, post-CDT, and 12 weeks after post-CDT) as the nested factor. Independent t-tests were performed to determine the difference in self-reported satisfaction between the two conditions in two groups (AL and LL, PL and LL). Pearson correlation analyses were conducted to determine associations between the reduction in PCEV and pre-CDT PCEV. All the collected data were analyzed using SPSS ver, 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Clinical characteristics
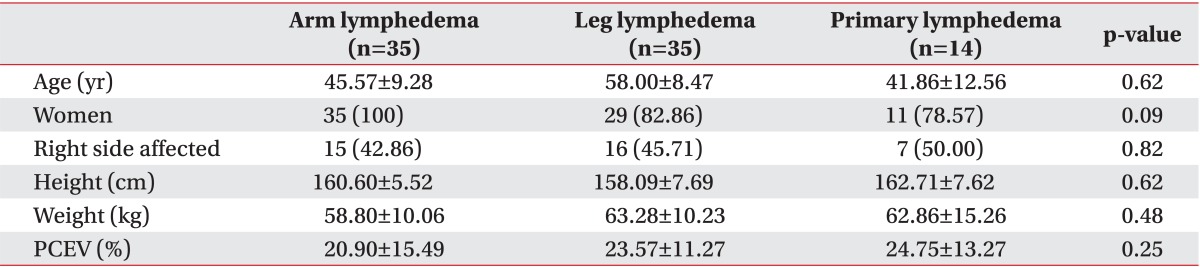
The patient characteristics are presented in Table 1. AL was present in 35 patients, and the remaining 49 patients had lower extremity lymphedema (LL and PL). With AL, only secondary lymphedema was present, and all patients had received prior treatment for breast cancer. Of the lower extremity patients, 14 had PL and 35 had LL. Of the LL patients, 19 (54%) patients had gynecologic cancer, 6 (17%) had prostate cancer, 5 (14%) had lymphoma, and 5 (14%) had other types of cancers. There were no differences in demographic characteristics among the AL, LL, and PL groups, except for a higher proportion of men in the lower extremity lymphedema (LL and PL) patients.
Volume
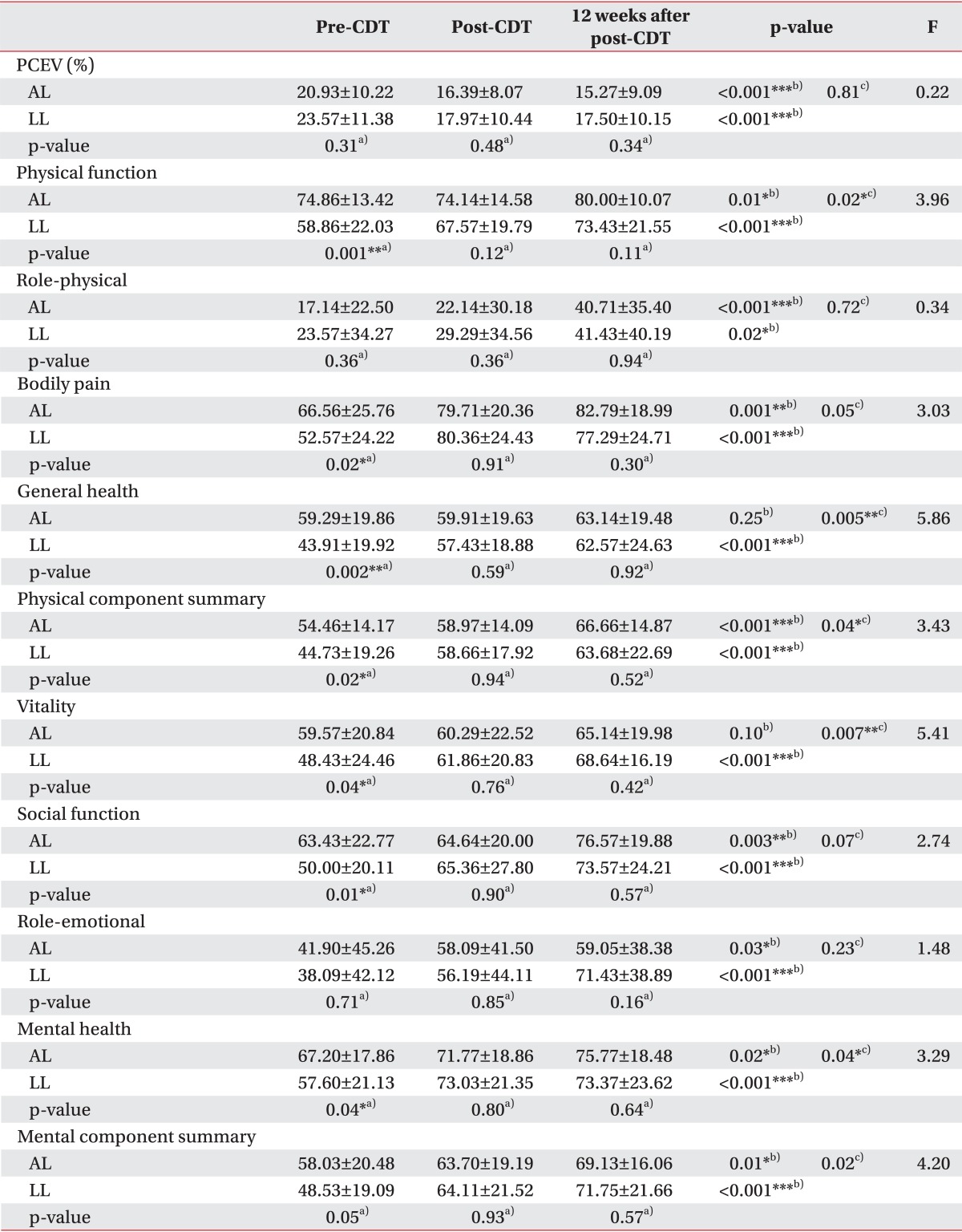
Mean baseline PCEV was 20.92% in AL, 23.57% in LL, and 24.75% in PL, relative to the normal limbs, and PCEV decreased post-CDT by 4.54% (21.69% of the pre-CDT PCEV; p=0.001), 5.61% (23.78% of the pre-CDT PCEV; p=0.001), and 6.00% (24.22% of the pre-CDT PCEV; p=0.01), respectively, compared with baseline (difference, F=0.31, p=0.73). At 12 weeks after treatment, the PCEV decreased by 5.66% (27.02% of the pre-CDT PCEV) in AL patients, 6.07% (25.75% of the pre-CDT PCEV) in LL patients, and 6.37% (25.74% of the pre-CDT PCEV) in PL patients, compared with baseline (difference, F=0.15, p=0.86). The differences in the change in PCEV between the groups were not significant at both post-CDT and 12 weeks after CDT (Tables 2, 3).

Changes in limb volume (%) and quality of life, as measured by the Short Form-36, at three time points before and after complex decongestive therapy by lymphedema type (PL and LL)
Changes in limb volume (%) and quality of life, as measured by the Short Form-36, at three time points before and after complex decongestive therapy by limb (AL and LL)
Patterns of volume reductions when expressed as PCEV remaining were similar between the groups. The initial 2 weeks represented the majority of PCEV reduction (Tables 2, 3). A statistically significant effect of time was evident from repeated measures analysis; however, this effect was restricted to the CDT sessions (p<0.001). The effects of the treatment and interaction of time by limbs or type were not significant (by limbs, p=0.96; by type, p=0.99). Because of these overall non-significant results for the primary outcome, subgroup analyses were not conducted.
The Pearson correlation analyses resulted in significant correlations between the reduction in PCEV (pre-CDT to post-CDT, and pre-CDT to 12 weeks after post-CDT) and pre-CDT PCEV (r=0.48, p<0.001; r=0.49, p<0.001, respectively).
Quality of life
In all groups, SF-36 scores were significantly lower than that of the Korean population means in almost all domains [2428]. There were no significant differences in the SF-36 scores for any of the time points between the PL and LL patients (p=0.69), but there were significant differences in the mean pre-CDT SF-36 scores between the AL and LL patients (p=0.01). The subjects reported a mean increase, from pre-CDT, of 10.23 points at post-CDT and 16.42 points at 12 weeks after post-CDT. Patients with LL showed more improved SF-36 scores than patients with AL (Table 3, Fig. 2).
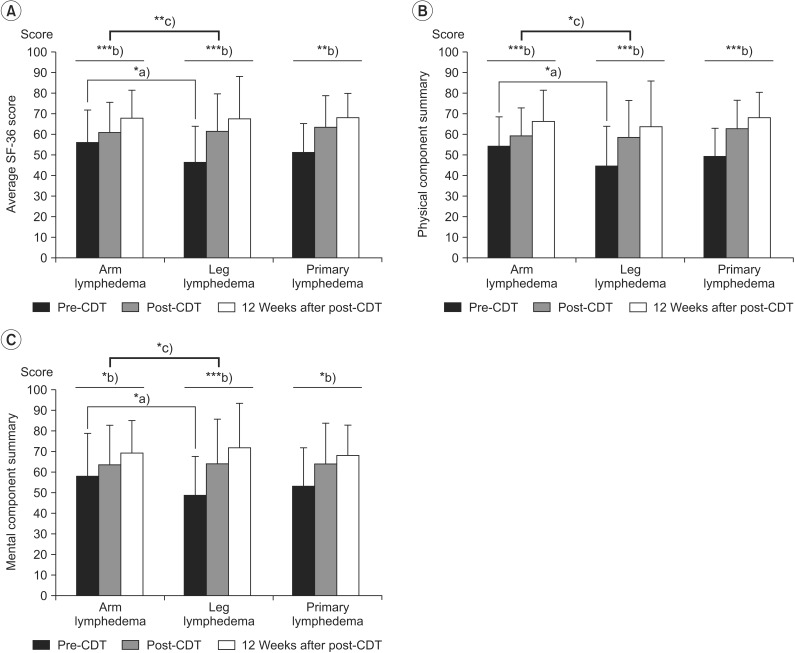
Changes in quality of life, based on the Short Form-36, in patients with lymphedema who underwent complex decongestive therapy (CDT). (A) Average SF-36 score, (B) physical component summary, and (C) mental component summary. *p<0.05, **p<0.01, ***p<0.001, a)p-values were derived from independent t-tests, b)p-values were derived from repeated measured ANOVAs for the effect of time for the within subject factor (3 levels: pre-CDT, post-CDT, and 12 weeks after post-CDT), c)p-values were derived from repeated measured ANOVAs for the effect of time for the between subjects factor (2 levels: AL and LL, PL and LL).
PF (p=0.001), VT (p=0.04), BP (p=0.02), SF (p=0.01), MH (p=0.04), and GH (p=0.002) scales were significantly lower in LL patients than in AL patients at pre-CDT. However, no significant difference was noted in the 6 items at post-CDT and 12 weeks after post-CDT. At the end of the observation period, most measures had improved, and there were no significant differences between AL and LL.
In the repeated-measures ANOVAs that were conducted with time (pre-CDT, post-CDT, and 12 weeks after post-CDT), a significant main effect for time between limb groups (AL and LL) was observed with the PF (F=3.96, p=0.02), GH (F=5.86, p=0.005), VT (F=5.41, p=0.007), and MH (F=3.29, p=0.04) scales.
Satisfaction survey
Satisfaction scores are reported in Fig. 3. The independent t-test with equivalent variance showed no significant differences between the PL and LL groups. The self-reported satisfaction scores from AL patients were lower than those from LL patients. Although no significant differences in volume reduction were observed between AL and LL, satisfaction was higher in LL patients.
DISCUSSION
Although several studies have focused on the overall deterioration in QOL over time, we are not aware of any previous study that has addressed differences in different limbs areas or lymphedema types. The current study evaluated the response of CDT characterized by 3 trajectories of type and limbs. Among 169 participants followed, 84 participants received efficacious CDT, and we tested the relationship between receiving CDT and the identified trajectories (AL, LL, and PL). The changing patterns of volume and QOL for all groups were examined for an identical period. Changes in all outcome measures were not significantly different between primary and secondary lymphedema. We found that the QOL of patients with LL was affected more than that of AL patients, and patients with LL experienced greater improvements in QOL and satisfaction with CDT.
The results of the present study are in close agreement with those of earlier studies that reported significant changes in edema volume and the mental and physical scales of the SF-36 over a 3-month period [5161727]. In all of the groups, the volume reduction primarily occurred within the 2 weeks of treatment, which is similar to previously reported findings [451826]. Further lymphedema volume reductions that were evident at least 12 weeks after treatment were not statistically significant (Fig. 4).
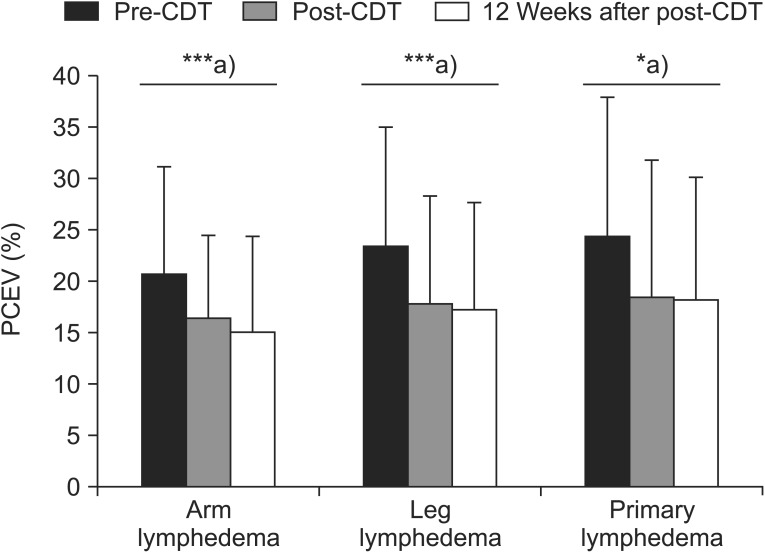
Changes in limb volume in patients with lymphedema, based on complex decongestive therapy (CDT). PCEV, percent excess volume. ***p<0.001, a)p-values were derived from paired t-tests.
The temporal improvement in edema volume was very similar in all groups, and a comparable magnitude of change occurred in all groups during the 14 weeks of the study. At 12 weeks after post-CDT, the edema volume had improved by 5.66% (27.02% of the pre-CDT PCEV), 6.07% (25.75% of the pre-CDT PCEV), and 6.37% (25.74% of the pre-CDT PCEV) in the AL, LL, and PL groups, respectively. The results of the current study correspond well with those of an earlier study which reported that volume differences were similar in patients with either upper- or lower-extremity lymphedema [29].
A previous study found that the most important predictor of the volume reducing effect of CDT was the amount of swelling at the time of presentation [183031]. Based on our experience, we expected that different reduction rates might be obtained based on the affected extremities and causes of lymphedema [32] and, similar to the aforementioned study, initial PCEV would affect CDT efficacy in both groups. However, there were no significant differences in the reduction rate of PCEV between the groups at any point during the trial. The current study showed that all groups produced similar changing patterns in PCEV, with CDT effectively promoting lymphedema volume reduction. This suggests that the affected limb or the cause is not correlated with the volume reductive effects of CDT, and the initial excessive volume rate results in similar changes in PCEV in both groups.
There were differences in QOL between patients with lymphedema in different limbs at baseline (pre-CDT). In the observation period, which included the CDT period, almost all of the SF-36 items showed statistically significant improvement in both groups. In a similar study assessing QOL outcomes after CDT for cancer lymphedema, the investigators found that CDT improved overall QOL and was effective for various degrees of lymphedema [4512]. Our analyses revealed the association of PCEV with SF-36. This association was markedly different in AL versus LL. Furthermore, CDT for LL resulted in greater treatment satisfaction. Because the clinical characteristics of AL such as cancer diagnosis, cancer treatment procedures and clinical courses may differ considerably from those of LL, we cannot generalize the results from the different effects of CDT. However our analyses revealed that differences in limbs might be predictive of changing patterns of QOL. Our findings suggest that the classification of types and limbs might be informative with regard to the aspect of QOL.
In our opinion, absolute PCEV may have a greater impact on QOL in patients with LL than in those with AL. However, it is not clear why lymphedema volume affects QOL more in those with LL than those with AL, although the impact on weight-bearing functions may provide an explanation. Volume reduction in the lower limb is essential for weight-bearing activities such as walking and transfer, which are included in SF-36 [16]. As such, these findings indicate early CDT is effective for lower-extremity lymphedema.
These results also can be useful in understanding the psychosocial impact of lymphedema. The observations of other studies show that significant psychosocial issues are associated with breast cancer related lymphedema more so than lymphedema from other causes [3334]. In our opinion, great improvements in physical and emotional health could be achieved by recognizing the psychosocial needs of patients with upper-extremity lymphedema. It would be interesting as well to explore the reasons for such responsiveness via exploration of the two groups (AL and LL).
To the best of our knowledge, no studies have been conducted to assess satisfaction with CDT using self-reported data in CDT. The degree of improvement, aesthetic satisfaction, and functional satisfaction were significantly more improved with LL than AL. This might be related to the greater improvement in the SF-36 score. Thus, CDT for volume reduction therapy might be the best treatment for patients with LL.
There were other findings that may be of clinical relevance. In general, patients with lymphedema responded differently depending on the type of lymphedema. In contrast to what is generally known, it has been reported that was no statistical significance between the outcomes of primary and secondary lymphedema groups in all measures [10]. In the present study, even though lymphedema had different causes, the response to CDT was similar in many ways. Despite differences in cause, onset age, disease duration and clinical course, we suggest that the type of lymphedema does not fully reflect the etiology, and the common physiopathological mechanism of primary and secondary lymphedema has to be identified [35]. In general, the degree of edema is correlated with the amount of lymphatic reserves. It may unlatch with CDT and the diminution of the volume is obtained. Thus, the response is determined by the amount of lymphatic reserves and the provision of therapeutic benefits by CDT in both type of lymphedema. It is suggested that the type of lymphedema (primary or secondary) is not able to predict the outcomes or additionally inform the treatment plan.
Our study was limited by several important factors. Our sample cannot be considered fully representative of patients with lymphedema because we excluded patients with severe systemic diseases, bilateral lymphedema, and severe lymphedema. Therefore, it is difficult to generalize these results. Second, a direct comparison of the upper and lower limbs is challenging. Although SF-36 was not specifically designed to compare arm and leg function directly, the robustness of the results is demonstrated by comparable results across instruments [17363738]. Finally, the absence of a control group limits the ability to attribute changes to CDT in both groups. These limitations suggest possible directions for future study.
Despite these limitations, the different outcomes in AL and LL patients support the theory that CDT is more beneficial for LL than AL, including those for QOL and satisfaction. The findings indicate a need for different approaches in management and patient consultation approaches for upper- and lower-extremity lymphedema. Knowledge of potential factors that influence CDT outcomes would help clinicians develop care plans. Intensive treatment for patients with LL is essential for the reduction of excessive volumes. As a result of CDT, reduction in the volume of lymphedema in lower extremity lymphedema can lead to improvements in QOL [530]. However, simple volume reduction therapy does not improve treatment satisfaction in patients with AL [13]. Future studies are warranted to investigate the factors that are associated with QOL in AL patients, given that it is not completely dependent on volume. Future research is needed to determine the related factors and plan therapeutic strategies in order to achieve the optimal status for QOL.
In the long-term, over 14 weeks, CDT was found to be effective in lymphedema, but with different longitudinal courses in QOL improvement and satisfaction with treatment according to the limb and type. We found that the pattern of presentation changed with CDT, with lower extremity lymphedema patients presenting more favorable outcomes in the aspects of QOL and satisfaction than upper extremity lymphedema patients. To sum up, clinicians should approach patients with different therapeutic considerations specific to each type or region of lymphedema. In patients with AL, simple volume reduction therapy does not improve satisfaction as well as QOL. Future clinical practice needs to detect LL early and address QOL through CDT. However, much more research remains to be done on the reasons for differences in responsiveness.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
Appendices
Appendix A
The study-specific satisfaction survey
A. Fill out demographic profile.
1. Demographics: Age, Height, Weight.
2. Underlying disease:
3. Lymphedema: onset, affected side.
B. Document your conditions.