Efficacy and Safety of High Density LED Irradiation Therapy for Patients With Hand Osteoarthritis: A Single-Center Clinical Study
Article information
Abstract
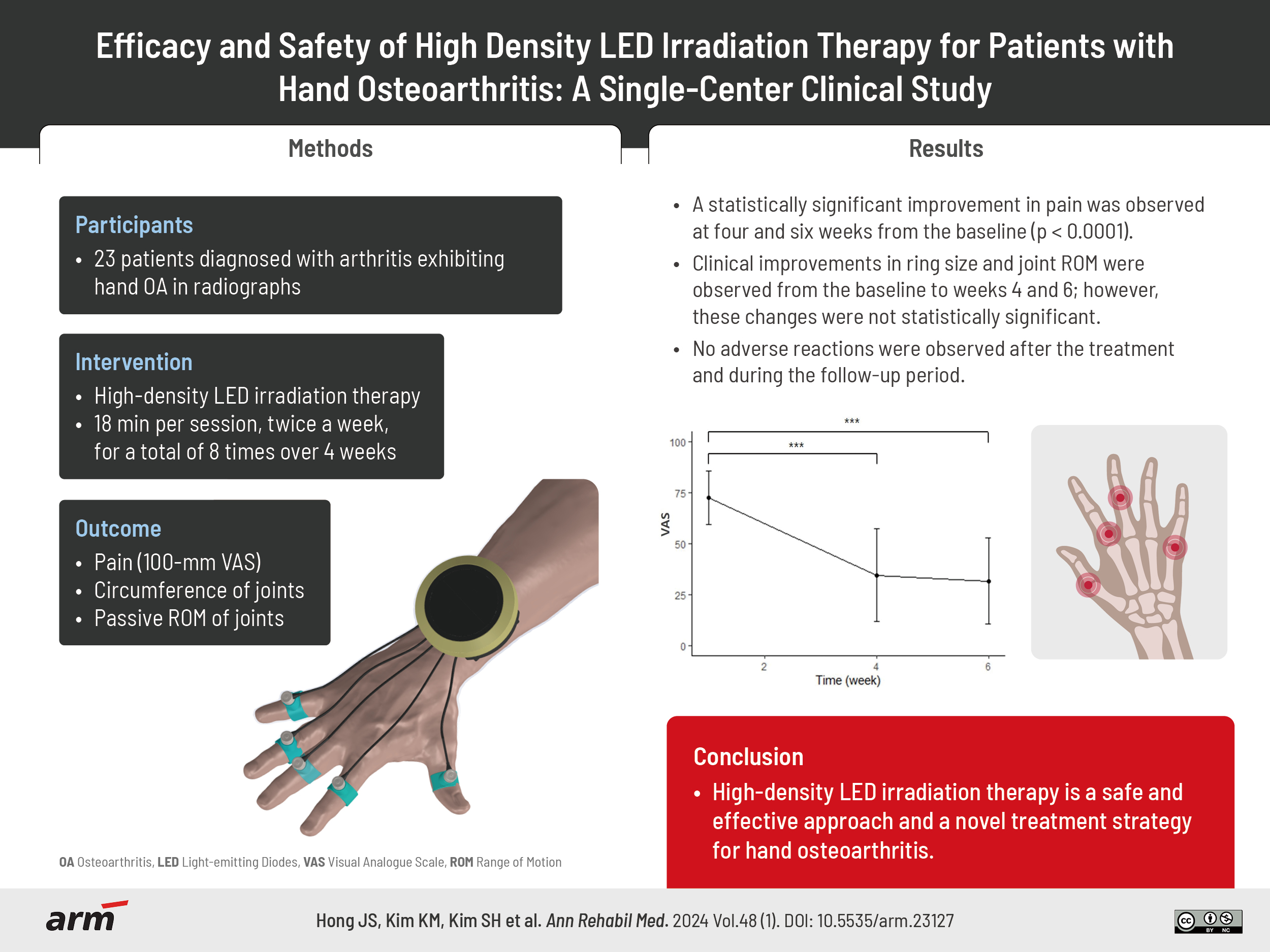
Objective
To assess the safety and effectiveness of high-density light-emitting diode (LED) irradiation therapy in patients with hand osteoarthritis (OA) and compare the pre- and post-intervention symptoms.
Methods
Twenty-three patients with hand OA underwent eight sessions of high-density LED irradiation therapy directed at the five most painful areas in the finger joints. Each session lasted for 18 minutes; and the sessions were conducted twice a week, for 4 weeks. We evaluated the degree of pain using the visual analogue scale, ring size, and passive range of motion (flexion+extension) for two most painful joints from the baseline to post-therapy (weeks 4 and 6).
Results
High-density LED irradiation therapy significantly reduced the pain posttreatment compared with that observed at the baseline (p<0.001). Although improvements were observed in ring size and joint range of motion at 4 and 6 weeks, they were not statistically significant (p>0.05). No adverse events were observed.
Conclusion
We examined the safety and effectiveness of high-density LED irradiation therapy in reducing pain and hand swelling and improving joint mobility in patients with hand OA. These results suggest that high-density LED irradiation therapy has the potential to be an important strategy for managing hand OA.
INTRODUCTION
Osteoarthritis (OA) is a musculoskeletal disease whose prevalence is rapidly increasing with the aging population and obesity trends [1,2]. OA is the fourth leading cause of nonfatal disability worldwide, with a large impact on medical and indirect costs due to work losses and premature retirement [3]. Patients with OA often experience significant limitations in their daily lives due to the severity of their illness. Unfortunately, damaged cartilage cannot regenerate, and reduced activity due to pain results in a decrease in synovial fluid volume, causing the affected joint to become stiff [4]. In the early stages, the pain tends to worsen when the joint is moved; however, as the disease progresses, it persists regardless of movement. Chronic pain is a hallmark of advanced arthritis and can significantly affect the quality of life [5]. As arthritis progresses, several characteristic symptoms appear, including decreased range of motion (ROM) of the joint, swelling, and tenderness around the affected joint [6]. The combined effects of these symptoms can lead to functional impairment and reduced mobility in patients with arthritis [7].
Hand OA can involve multiple joints, such as the distal and proximal interphalangeal joints, and presents with various patterns [8]. Patients with hand OA have weak grip strength, poor accuracy, and poor fine motor skills, which not only cause loss of work capacity but also pose many obstacles to their daily lives. Despite its high prevalence and complications, previous studies have focused only on arthritis of large joints such as the hips and knees, and research on arthritis of small joints such as those of the hands is lacking [9,10].
The primary goal of hand OA treatment is to alleviate the associated symptoms. Treatment modalities for hand OA include non-pharmacological approaches such as ROM training, muscle strengthening exercises, utilization of supportive devices, and the application of splints [11]. Pharmaceutical interventions involve the use of acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs) orally or topically. The European League Against Rheumatism has proposed an integrated approach that combines both non pharmacological and pharmacological treatments to effectively manage hand OA [12]. However, among the representative pharmacological therapies, therapy using NSAIDs may adversely affect the digestive system and blood coagulation mechanism [13]. In addition, the opioids used for pain relief may induce sedation, mood fluctuations, depression, and increased anxiety, combined with drug dependence [14].
In some cases, medical professionals administer intra-articular injections of corticosteroids or hyaluronic acid into the affected joints to reduce symptoms and improve mobility. However, these effects are temporary and may promote articular cartilage degeneration and infection, potentially impeding disease progression [15]. Consequently, the long-term effectiveness of intra-articular therapies in the management of hand OA is limited [16]. Moreover, the 2012 American College of Rheumatology (ACR) guidelines do not recommend this approach [17].
Surgical procedures such as arthroscopic debridement, synovectomy of the trapeziometacarpal joint, and early tendon arthroplasty are typically considered the last option for the treatment of hand OA and are pursued only when less invasive treatments have proven ineffective [18]. Because surgical interventions are highly invasive, complications such as pain, infection, instability, nerve dysfunction, tendon-pulling sensation, and chronic regional pain syndrome are common. Furthermore, the patients need to undergo postoperative rehabilitation [19].
Photobiomodulation therapies such as low-power laser therapy and light-emitting diode (LED) therapy have been proposed as novel treatments for skin cancer and pain relief [20,21]. They have the advantage of being safe, non-addictive, and non-invasive without side effects [21]. LEDs influence cellular metabolism by initiating intracellular photobiochemical reactions. The notable outcomes include increased adenosine triphosphate production, modulation of reactive oxygen species, reduction of pro-inflammatory cytokines like interleukin (IL)-1β, IL-6, and tumor necrosis factor-α, activation of transcription factors, changes in collagen synthesis and angiogenesis, and improvements in blood circulation [22]. Prior research has demonstrated the efficacy of LEDs in reducing inflammation, pain, and edema through these mechanisms [20,23]. Owing to their relatively low cost and safety compared with that of lasers, LEDs are used worldwide in various fields. Despite these advantages, research on the effects of LEDs on musculoskeletal problems is insufficient.
In this study, we aimed to determine the safety and efficacy of high-density LED irradiation therapy in patients with hand OA and compare the patients’ symptoms before and after the intervention.
METHODS
Participants and study design
This single-group study was performed between July and August 2023 at a single clinical center. Individuals aged between 40 and 80 years, diagnosed with arthritis according to the ACR guidelines, exhibiting hand OA in anteroposterior radiographs, and with a visual analogue scale (VAS) score of 40 mm or greater at the screening visit were included.
Patients who had undergone hand-joint surgery, experienced pain in areas other than the hand joint, or had ligament instability of >5 mm were excluded. Individuals who had taken NSAIDs within 48 hours before screening, immunosuppressive medications within 6 weeks, psychotropic or narcotic analgesics within 8 weeks, or intra-articular injections within 6 months were excluded.
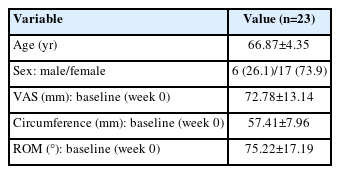
A total of 23 patients participated in the study. We evaluated the degree of pain in the hand at rest using a 100-mm VAS and circumference and passive ROM of the two most painful joints at the baseline, based on the design of the single previous study on irradiation therapy for hand OA, in which an average of 2.50±1.54 joints per patient were examined [9]. The VAS score was assessed after 10 minutes of rest in the sitting position. According to the neutral-zero method, the zero position of a joint is defined as the normal anatomical position [24]. The ROM was determined by adding the flexion and extension angles. The measurements were obtained by an expert in the field of Physical Medicine and Rehabilitation. After eight treatment sessions lasting 4 weeks, the three outcome measures were re-evaluated for all patients.
The study protocol was approved by the Institutional Review Board of Yonsei University Wonju Severance Christian Hospital (No. CR222026). All the participants provided written informed consent. The investigators explained the purpose, methods, and potential risks of the study to all the participants.
High-density LED irradiation therapy
A high-density LED irradiation therapy device (PT-100/iPHOTON; MI.One) was used to treat the five most painful interphalangeal joints (Fig. 1). The device was equipped with infrared LEDs having wavelengths of 850 and 940 nm. High-density light irradiation was applied for 18 minutes per session, twice a week, for a total of eight times over 4 weeks.
Safety assessment
Adverse events were evaluated for each treatment session up to 30 minutes after the end of the application. Vital signs (systolic/diastolic blood pressure, pulse rate, and body temperature) were recorded. The patients were asked to spontaneously report information on adverse events such as pain, erythema and blistering and other discomfort they felt, as needed. Patient diaries were prepared, and interviews with the patients were conducted throughout the study period.
Statistical analysis
To analyze the results of this study, the SAS software version 9.4 (SAS Institute Inc.) and R Studio software version 4.1.3 were used. Descriptive statistics (including mean, standard deviation, median, and minimum and maximum values) were used to examine the changes in patient outcome measurements. This assessment was conducted using a paired t-test with the baseline hand pain value considered as a covariate.
We used the repeated measures ANOVA technique and performed Bonferroni analysis for multiple comparisons. Differences among groups in terms of continuous variables were evaluated using one-way ANOVA, and post hoc comparisons were adjusted using the Bonferroni method. For dichotomous variables, χ2 tests were performed. The relationships between continuous variables were analyzed using Pearson’s correlation coefficients. For all tests, statistical significance was set at a p-value of less than 0.05.
RESULTS
A total of 23 patients of 6 males and 17 females were enrolled in the study, and a significant difference was observed between the sexes (p=0.0218). The mean age of the patients was 66.87±4.35 years, and the baseline VAS was 72.78±13.14 mm. The baseline circumference of 46 joints (two joints in 23 patients) was 57.41±7.96 mm and the degree of ROM was 75.22°±17.19° (Table 1).
The post hoc analysis using Bonferroni for multiple comparisons among the three time points revealed a significant difference between the VAS scores at the baseline and the 4 weeks posttreatment, as well as between the scores at the baseline and 6 weeks posttreatment (p<0.0001). However, no statistically significant difference was observed between the scores obtained at 4 and 6 weeks posttreatment (p=0.59). Although the ring sizes and joint ROMs improved at 4 and 6 weeks from the baseline, these changes were not statistically significant (p>0.05; Table 2, Figs. 2-4).
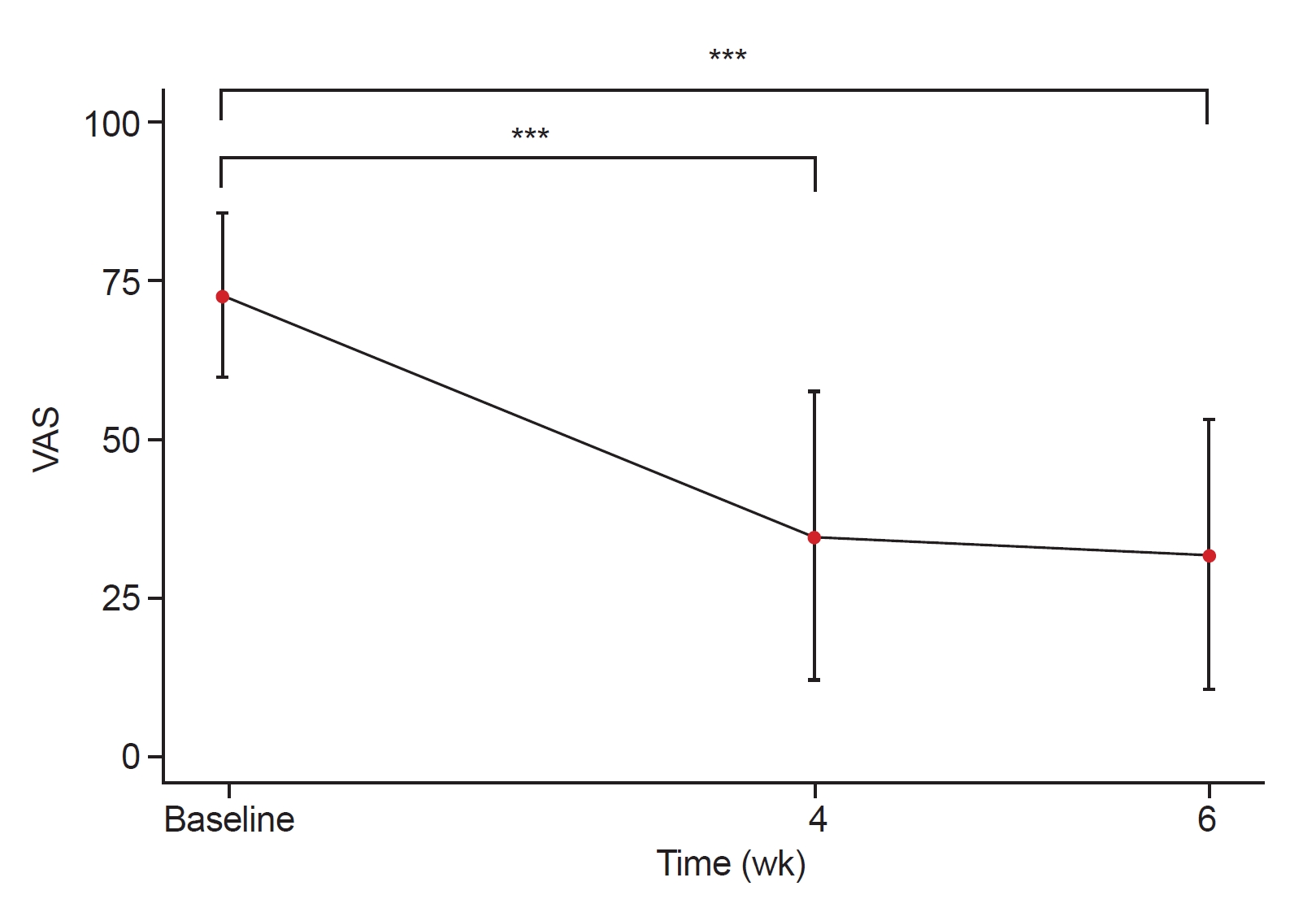
Changes in mean visual analogue scale (VAS; 0 mm=no pain, 100 mm=most severe pain) scores at baseline (week 0), after week 4 and 6 over time error bars indicate standard deviations, p-values were calculated by one-way within subjects ANOVA’s and Bonferroni corrected post hoc comparisons.
***p<0.0001.

Changes in ring size at baseline (week 0), after week 4 and 6 over time error bars indicate standard deviations.

Changes in joint passive range of motion (PROM, flexion+extension) baseline (week 0), after week 4 and 6 over time error bars indicate standard deviations.
No adverse reactions were observed after the treatment and during the follow-up period.
DISCUSSION
This is the first study in which the efficacy and safety of high-density LED irradiation therapy for pain relief, hand edema, and improved joint ROM was evaluated in patients with hand OA.
A statistically significant improvement in pain was observed at 4 and 6 weeks from the baseline (p<0.0001); however, no significant difference was observed between the symptoms at these two intervals (p=0.59). The treatment resulted in significant pain reduction after 4 weeks, and the delayed effect lasted for two weeks. Clinical improvements in ring size and joint ROM were observed from the baseline to weeks 4 and 6; however, these changes were not statistically significant (p>0.05). It is possible that the participants exhibited high initial VAS scores but relatively mild edema and limited ROM. Moreover, there was a significant enhancement in the VAS scores was evident after the LED irradiation therapy. Consequently, we observed improvement of circumference or passive ROM plausibly indicative of clinical progress without any statistically significant outcomes. Therefore, further studies focusing on cases with severe edema and limited ROM are warranted.
Previous studies on LED irradiation therapy have shown that prolonged irradiation can cause treatment-site pain, erythema, hyperpigmentation, and blistering. However, most patients recover without permanent injuries [25,26]. The safety evaluation revealed the absence of any adverse events after the application of high-density LED irradiation therapy, and the risk associated with high-density LED irradiation therapy devices was considered low. In several other studies conducted using LEDs for skin lesions, the side effects were either mild or not reported [21,23].
The depth of tissue penetration depends on the tissue type. In general, penetration depths are as follows: less than 1 mm at 400–470 nm, 0.5–2 mm at 570–590 nm, 2–3 mm at 630–700 nm, and 5–10 mm at 800–1,200 nm [21]. The longer the wavelength, the higher is the penetration. Several studies have highlighted that combining LED wavelengths is more effective than using a single wavelength to treat skin lesions [22,23,27]. Hence, we opted for infrared wavelengths to reach the joint effectively and chose a combination of LED wavelengths.
Various treatments, including medications, physical therapy, and surgery, have been used to treat OA, but their use has been limited due to several disadvantages such as multiple organ damages from NSAIDs [13], the risk of opioid abuse [14], and infectious arthritis and cartilage damage from intra-articular injections [15]. Therefore, noninvasive light therapies such as lasers and LEDs have recently emerged as treatments for musculoskeletal problems. Although studies have been conducted on the improvement of pain, inflammation, and edema using low-level lasers, research on LED therapy is lacking [28-32]. In addition, LED light therapy is not only safer than laser therapy, which is associated with side effects (eye and skin irritations), but also has numerous advantages: convenience of utility at home, application to a wider range of target tissue areas, wearable feature, and the considerably lower cost than laser therapy [33].
Limitations
Our study had several limitations. Firstly, because it was not a randomized controlled trial, we could only compare the symptoms of the patients pre- and post-therapy and could not examine untreated versus treated patients. Additionally, we cannot completely rule out the possible influence of placebo effects. Secondly, the study had a limited sample size and a significant sex imbalance, with a higher proportion of female than that of male. Thirdly, we examined a relatively short-term effect for approximately 6 weeks; and thus, the long-term effect could not be verified. Finally, since the LED therapy was applied only to patients with OA of the hand, verification of the therapy’s effectiveness is difficult in patients with OA affecting other parts of the body.
Conclusions
LED irradiation therapy showed to have effect on pain relief based on the reported results among our study participants. This study suggests that high-density LED irradiation therapy is a safe and effective approach and a novel treatment strategy for hand OA.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING INFORMATION
This research was funded by the Ministry of SMEs and Start-ups of the Republic of Korea (grant number: S3090288).
AUTHOR CONTRIBUTION
Conceptualization: Kim K, Kim SH, Hong J. Methodology: Kim K, Choi WW, Hong J, Kang DR, Hong S. Formal analysis: Kim K, Kim JH, Hong J. Funding acquisition: Kim SH. Project administration: Kim K, Kim SH, Hong J. Visualization: Kim K, Yong SY, Kim SJ, Hong J. Writing – original draft: Kim K, Hong J. Writing – review and editing: Kim K, Kim SH, Kim HD, Oh KJ, Hong J. Approval of final manuscript: all authors.