Sarcopenic Dysphagia and Simplified Rehabilitation Nutrition Care Process: An Update
Article information
Abstract
Sarcopenic dysphagia is characterized by weakness of swallowing-related muscles associated with whole-body sarcopenia. As the number of patients with sarcopenia increases with the aging of the world, the number of patients with sarcopenic dysphagia is also increasing. The prevalence of sarcopenic dysphagia is high in the institutionalized older people and in patients hospitalized for pneumonia with dysphagia in acute care hospitals. Prevention, early detection and intervention of sarcopenic dysphagia with rehabilitation nutrition are essential. The diagnosis of sarcopenic dysphagia is based on skeletal and swallowing muscle strength and muscle mass. A reliable and validated diagnostic algorithm for sarcopenic dysphagia is used. Sarcopenic dysphagia is associated with malnutrition, which leads to mortality and Activities of Daily Living (ADL) decline. The rehabilitation nutrition approach improves swallowing function, nutrition status, and ADL. A combination of aggressive nutrition therapy to improve nutrition status, dysphagia rehabilitation, physical therapy, and other interventions can be effective for sarcopenic dysphagia. The rehabilitation nutrition care process is used to assess and problem solve the patient’s pathology, sarcopenia, and nutrition status. The simplified rehabilitation nutrition care process consists of a nutrition cycle and a rehabilitation cycle, each with five steps: assessment, diagnosis, goal setting, intervention, and monitoring. Nutrition professionals and teams implement the nutrition cycle. Rehabilitation professionals and teams implement the rehabilitation cycle. Both cycles should be done simultaneously. The nutrition diagnosis of undernutrition, overnutrition/obesity, sarcopenia, and goal setting of rehabilitation and body weight are implemented collaboratively.
INTRODUCTION
Sarcopenia, defined as loss of muscle strength, muscle mass, and physical function, is increasing with the aging population [1]. According to the World Health Organization report, the percentage of the world population aged 65 and over is projected to increase from 5% in 1950 to 10% in 2022 and 16% by 2050 [2]. Aging is associated with a wide range of aging phenomena. Handgrip strength increases with age, peaking in the 30s and 40s, then declines thereafter [3,4]. Muscle mass increases with age, then maintains or decreases after the 40s and 50s [3,5,6]. The number of comorbidities increases with age in older people [7], and they suffer from polypharmacy. Polypharmacy is a risk factor for sarcopenia [8]. Sarcopenia is associated with adverse outcomes of falls, fractures, mortality, decreased Activities of Daily Living (ADL), dysphagia, dyspnea, and decreased quality of life [9-11]. Therefore, treatment and prevention of sarcopenia are essential.
Poor swallowing muscle mass and strength and swallowing function worsen outcomes such as malnutrition, aspiration pneumonia, prolonged hospitalization, and survival in older people [12]. Many muscles are involved in swallowing, such as the tongue, geniohyoid, mylohyoid, digastric, stylohyoid, and temporal muscles. Unlike skeletal muscles, the geniohyoid muscle is a striated muscle, and its histological characteristics make it difficult for swallowing muscles to atrophy [13]. However, the geniohyoid muscle volume and cross-sectional area of the tongue decrease with age [14,15]. The prevalence of dysphagia in older people is approximately 20%–35%, although it varies by countries and regions [16,17]. The prevalence increases further in the presence of dementia and cerebrovascular diseases. Community-dwelling older people may be at risk for malnutrition and poor swallowing function [18], and hospitalized and institutionalized adults are at increased risk for malnutrition due to poor swallowing function [19]. Many patients hospitalized for community-acquired pneumonia may have dysphagia [20]. As many as 75% of aspiration pneumonia patients over the age of 65 have dysphagia, and dysphagia increases hospital stay by 3.5 days and mortality by 1.7 times [21]. The association of swallowing muscles and swallowing function/severity assessment with frailty and sarcopenia has been reported [22,23].
Sarcopenic dysphagia is due to whole-body sarcopenia and low muscle mass and strength related to swallowing [13]. Decreased limb and trunk skeletal muscle mass involved in postural retention leads to decreased swallowing muscle mass [14,23]. In a study of the effects of food abstinence in older hospitalized patients, 77% had sarcopenia and 26% had dysphagia after two months [24]. This development of dysphagia was related to the effects of bed rest and no oral intake during hospitalization. Effective intervention with rehabilitation nutrition can lead to the prevention of sarcopenic dysphagia [25,26].
Rehabilitation nutrition is defined as that (1) a holistic assessment using the International Classification of Functioning, Disability and Health and evaluation of the cause of malnutrition, sarcopenia, and excess or deficient nutritional intake, (2) diagnosis and goal setting through rehabilitation nutrition, (3) to improve the nutrition status, sarcopenia, frailty and disability to practice nutritional management from rehabilitation and rehabilitation from nutrition to maximize their function and quality of life [27].
Evidence on rehabilitation nutrition was gradually accumulating, and the 2020 Clinical Practice Guidelines for Rehabilitation Nutrition weakly recommend enhanced nutrition therapy in cerebrovascular disease, hip fracture, cancer, and acute illness [28]. The Japanese Association of Rehabilitation Nutrition have been reported 7 position papers, including “Goal setting for nutrition and body weight in rehabilitation nutrition” [29], “Nutritional, physical therapy for specific diseases” [30] and “Respiratory sarcopenia: a position paper by four professional organizations” [31].
With medical advances in today’s aging population, the number of people with sarcopenia and frailty are expected to increase. The challenge is how to increase healthy life expectancy. Sarcopenia, dysphagia and oral frailty affect quality of life [32-34]. Early identification of patients with sarcopenic dysphagia and effective prevention and treatment strategies are important. This review describes the diagnosis, prevalence, epidemiology, treatment and prevention, and rehabilitation nutrition of sarcopenic dysphagia and the simplified rehabilitation nutrition care process.
SARCOPENIC DYSPHAGIA
Diagnostic criteria and screening
Diagnostic criteria for sarcopenic dysphagia are based on loss of skeletal and swallowing-related muscle mass and strength [35]. According to Wakabayashi [36], Diagnostic criteria for sarcopenic dysphagia is as follows: (1) Presence of dysphagia. (2) Presence of whole-body sarcopenia. (3) Results of imaging studies are consistent with loss of swallowing muscle mass. (4) Causes of dysphagia other than sarcopenia are excluded. Ultrasound, computed tomography, and magnetic resonance imaging can evaluate swallowing-related muscle mass [37-39]. In particular, ultrasound evaluation of the geniohyoid muscle shows good reliability and validity [40,41].
The diagnostic flowchart of sarcopenic dysphagia does not include an assessment of swallowing-related muscle mass [42]. Patients 65 years of age and older with no cognitive decline are evaluated and diagnosed for physical function, muscle mass, swallowing function, underlying disease, and strength of swallowing-related muscle (Fig. 1). Physical function and total body muscle mass are determined using the diagnostic criteria of the European Working Group on Sarcopenia in Older People 2 [10] or the Asian Working Group for Sarcopenia (AWGS) 2019 [43]. The cut-off values for hand grip strength are determined by male≤28 kg, female≤18 kg, or gait speed<1.0 m/sec in the AWGS 2019 diagnostic criteria. Assessment of swallowing function includes the revised Modified Water Swallowing Test, Water Swallowing Test, Food Test, Video Endoscopic Examination of Swallowing, and Video Fluoroscopic Examination of Swallowing. Muscle strength of the swallowing-related muscle is assessed by tongue pressure [44-52]. A diagnosis of probable sarcopenic dysphagia is made if there is muscle weakness in tongue pressure (<20 kPa). If there is no weakness in tongue pressure or if tongue pressure is difficult to measure, a diagnosis of possible sarcopenic dysphagia is made.
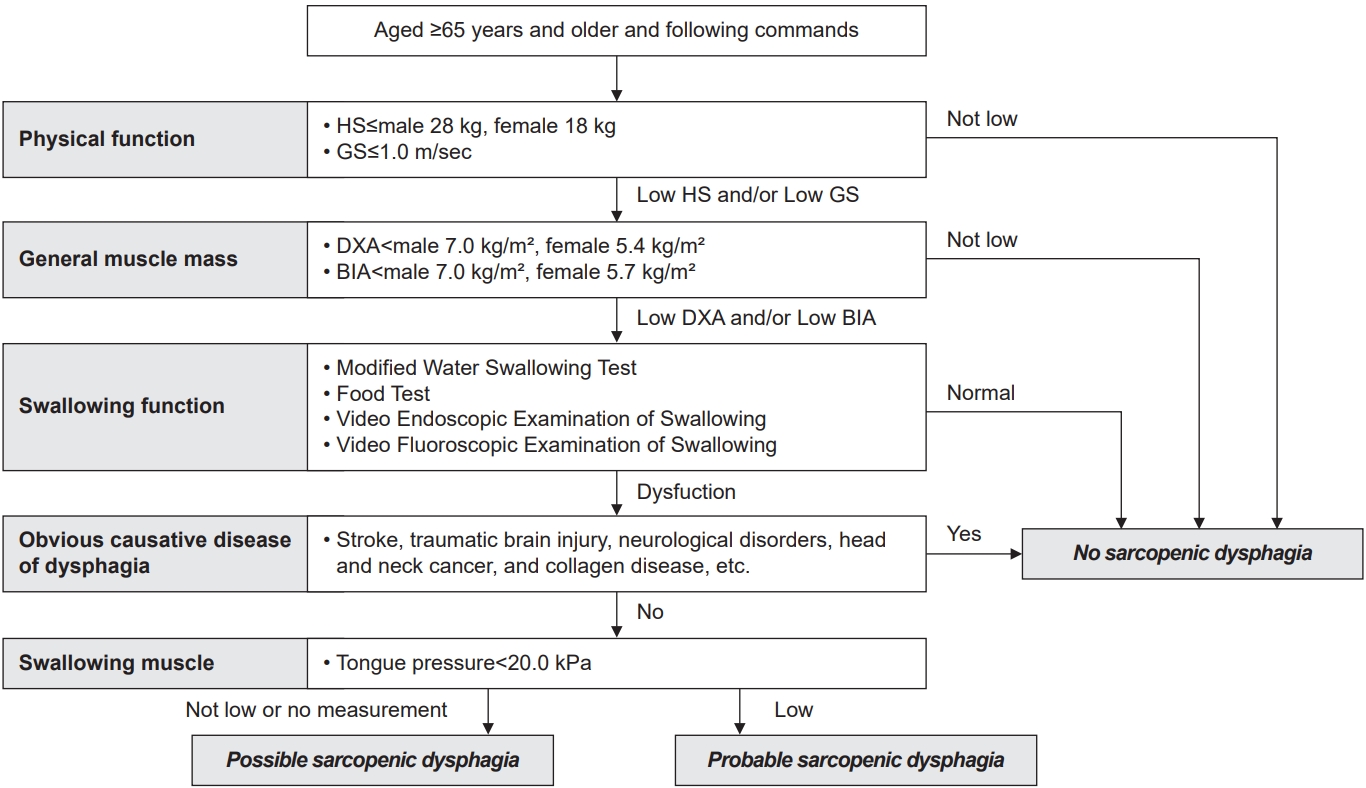
Diagnostic flowchart of sarcopenic dysphagia. HS, hand grip strength; GS, gait speed; DXA, dual-energy X-ray absorptiometry; BIA, bioelectrical impedance analysis.
Screening for sarcopenic dysphagia includes handgrip strength, calf circumference, or body mass index. The cut-off values were 19.7 kg for handgrip strength in males and 29.5 cm for calf circumference in females [53]. The body mass index cut-off value was 20.1 kg/m2 in older dysphagia patients [54]. Early assessment of swallowing function may prevent sarcopenic dysphagia. Therefore, early screening and using the diagnostic flowchart is recommended [13].
Prevalence
Sarcopenic dysphagia has a high prevalence among institutionalized older people and patients hospitalized for pneumonia with dysphagia in acute care hospitals. Of 100 sarcopenic seniors aged 65 years and older in nursing homes, 45% had sarcopenic dysphagia [55]. In an acute general hospital, 32% of patients undergoing swallowing rehabilitation had sarcopenic dysphagia [56]. Among patients hospitalized for pneumonia with dysphagia, 81% had sarcopenic dysphagia [57]. Patients with lower skeletal muscle mass and grip strength at baseline <8 kg had a higher incidence of dysphagia at discharge in postoperative hip fracture inpatients [58]. Dysphagia is common in patients with older age, poor performance status, gait disturbance, low body weight, malnutrition, and poorer food intake [59]. Sarcopenic dysphagia in community-dwelling older people is underreported and its prevalence is unknown.
Prognosis
Patients with sarcopenic dysphagia have poor improvement in swallowing function and are prone to malnutrition [60]. In addition, patients with malnutrition have significantly weaker swallowing-related muscles, which can easily lead to a vicious cycle of further deterioration of swallowing function compared to patients with normal nutrition status [61]. Patients with sarcopenic dysphagia have less improvement in swallowing function than patients with non-sarcopenic dysphagia [60]. From a nutritional standpoint, sarcopenic dysphagia is associated with poor improvement in swallowing function because they tend to be malnourished. On the other hand, swallowing function improves better with swallow muscle strengthening interventions in patients with sarcopenic dysphagia compared to patients without sarcopenic dysphagia [62]. From a physiological perspective, targeting the swallowing muscles directly seems effective in improving swallowing function because they do not suffer from damage caused by diseases such as stroke and neck cancer.
Sarcopenic dysphagia is associated with increased mortality and poor ADL improvement in older people. Malnutrition is associated with muscle weakness, wasting, physical frailty, and complications during hospitalization, and impedes improvement in ADL and increases mortality [61,63]. Mortality is 1.4 times higher among older people with sarcopenic dysphagia than among those without dysphagia in care facilities [62]. The risk of death is higher, especially when they are affected by weight loss and malnutrition [64]. Patients with sarcopenic dysphagia tend to have low ADL independence and poor improvement compared to patients with non-sarcopenic dysphagia [60]. Moreover, ADL improvement is significantly lower in malnourished patients compared those with normal nuti status.
High levels of inflammation are associated with poor improvement in swallowing function, and C-reactive protein (CRP) may be a prognostic predictor of swallowing function in patients with sarcopenic dysphagia [64]. Mori et al. [64] defined patients with CRP of ≥5.0 mg/L as the high-inflammation group and reported poor improvement in swallowing function in the high-inflammation group compared to the low-inflammation group. Adding the result of CRP levels to the initial assessment of swallowing function could predict the more accurate prognosis.
Treatment and prevention
The combination of aggressive nutrition therapy and rehabilitation can effectively treat sarcopenic dysphagia [13,25]. Aggressive nutrition therapy is nutritional care management that sets goals for total daily energy intake according to daily energy expenditure and daily energy accumulation [35]. Older patients require more than 250 kcal/day of daily energy accumulation to gain 1 kg of body weight per month [65]. A mean provided energy intake of ≥30 kcal/ideal body weight (kg)/day improved swallowing function in older patients with dysphagia, and ADL improved significantly in the patient undergoing rehabilitation in the acute care hospital [66]. Treatment of sarcopenic dysphagia could improve muscle strength and function throughout the body, including the swallowing muscles, by combining aggressive nutrition therapy, dysphagia rehabilitation, and physical interventions such as physical therapy [66-68].
Hospitalized patients need to evaluate and monitored to minimize sarcopenic dysphagia. Hospital-associated sarcopenia and acute sarcopenia are classified into iatrogenic and non-iatrogenic [69]. While prevention of non-iatrogenic sarcopenia is difficult, prevention of iatrogenic sarcopenia caused by the medical activities of health care professionals including doctors, nurses, and others is possible. The combination of rehabilitation, appropriate nutritional management, and medication review from admission may prevent sarcopenia during hospitalization [69].
Early detection and intervention are necessary because older people with presbyphagia are at risk for sarcopenic dysphagia. Presbyphagia is characterized by the fragility of the swallowing mechanism due to age-related changes [70-75]. Presbyphagia in older people could develop into sarcopenic dysphagia because of their disease, activity level, and nutrition status when hospitalized for diseases [36]. Monitoring nutrition status and swallowing function in older people by questionnaire such as the 10-item Eating Assessment Tool and clinical examination may be effective in preventing sarcopenic dysphagia during hospitalization [76-81].
REHABILITATION NUTRITION
Rehabilitation nutrition care process
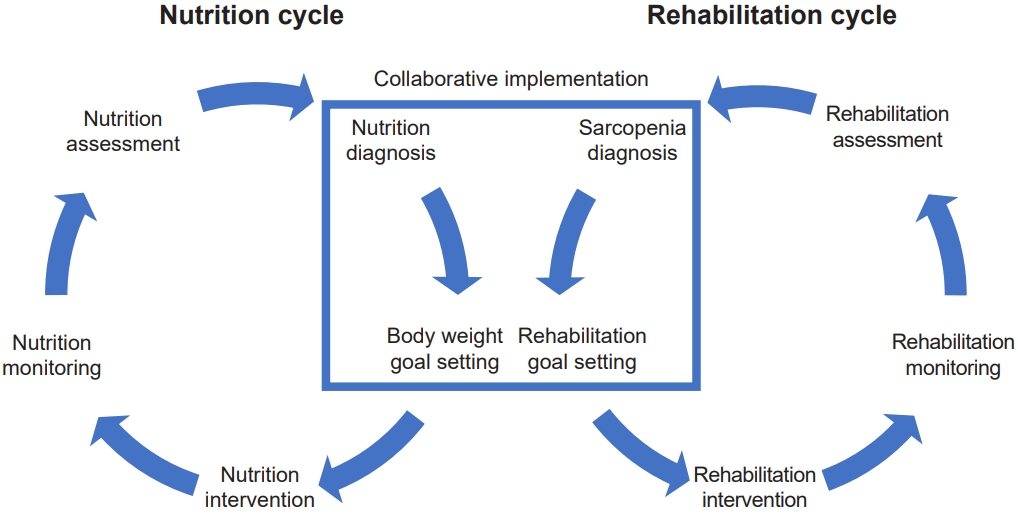
The rehabilitation nutrition care process is a systematic problem-solving method used to assess the nutrition status, sarcopenia, and nutritional intake of people with disabilities and frail older people [82]. The rehabilitation nutrition care process consists of five steps [83]: (1) rehabilitation nutrition assessment and diagnostic reasoning, (2) rehabilitation nutrition diagnosis, (3) rehabilitation nutrition goal setting, (4) rehabilitation nutrition intervention, and (5) rehabilitation nutrition monitoring. Collaboration between rehabilitation and nutrition helps set appropriate goals and develop appropriate rehabilitation and nutrition care management [26].
The simplified rehabilitation nutrition care process consists of a nutrition cycle and a rehabilitation cycle, each with five steps. Nutrition professionals and teams implement the nutrition cycle (Fig. 2). Rehabilitation professionals and teams implement the rehabilitation cycle. Goal setting is based on diagnostic reasoning about the presence and cause of malnutrition and sarcopenia. In addition, rehabilitation and nutrition professionals should work together to achieve rehabilitation and nutrition goals. The nutrition diagnosis of undernutrition, overnutrition/obesity, sarcopenia, and goal setting of body weight and rehabilitation should be implemented collaboratively. Both cycles should be done simultaneously. The physiatrists can conduct both cycles simultaneously.
Diagnostic reasoning in rehabilitation nutrition
Diagnostic reasoning is the thought process that leads to a diagnosis based on symptoms, laboratory findings, and test results [84]. Diagnostic reasoning is a fundamental skill for healthcare professionals, because the correct diagnosis of a condition leads to the appropriate treatment. Inadequate diagnostic reasoning about nutrition status and sarcopenia would cause an inappropriate rehabilitation nutrition. Therefore, proper diagnostic reasoning is required to provide effective rehabilitation nutrition.
The thought process in diagnostic reasoning consists of non-analytic (intuitive) and analytic reasoning and requires taking advantage of them properly. Non-analytic reasoning is a diagnostic method that quickly understands and recognizes disease patterns based on experience. Intuitive, automatic, and fast are the main attributes of this method, and experienced medical professionals often use it appropriately [85]. However, it is susceptible to bias and carries the risk of misdiagnosis. Analytic reasoning is a logical diagnostic method that is time-consuming and requires more resources than the other [86]. Analytic reasoning is often used by novices with limited clinical experience. Balancing both methods without showing bias toward one or the other is important for proper diagnosis.
Among the various nutritional problems, diagnostic reasoning in rehabilitation nutrition is critical in three of them [84]: causes of anorexia, weight loss, and sarcopenia. The causes of anorexia, weight loss, and sarcopenia are not necessarily a single cause, but rather multiple causes. Therefore, analytical reasoning is recommended in these conditions to infer these causes.
Goal setting in rehabilitation nutrition
Goal setting in rehabilitation nutrition is based on SMART, an effective goal setting method to improve goal attainment [87]. This method recommends incorporating five elements: Specific, Measurable, Achievable, Relevant, and Time-Bound [87]. For example, gaining 2 kg of body weight in one month and being able to walk independently indoors with a walking cane in two weeks are considered SMART goals. Goal setting based on SMART affects the rate of goal attainment. In a study of patients with stage 3–4 chronic kidney disease, food intake was higher in the SMART goal-setting group than in the non-SMART goal-setting group [88]. Setting weight goals makes rehabilitation nutrition interventions more appropriate, whether to gain weight in underweight patients or to lose weight in overweight patients. In obese patients undergoing cardiac rehabilitation, setting a weight loss goal results in greater weight loss than not setting a goal [89].
Aggressive nutrition therapy
Aggressive nutrition therapy is a nutritional management method for malnutrition [35]. Aggressive nutritional therapy must be combined with active exercise and rehabilitation to increase muscle mass and strength. The target energy intake in this method is defined as the sum of the total energy expenditure and the daily energy accumulation used to gain or lose body weight intentionally. The energy requirements for weight gain in patients with malnutrition and sarcopenia depend on a variety of patient factors, including sex, age, nutrition status, functional status, changes in body composition, contents of exercise, systemic inflammation, and comorbidities.
The energy requirements are set by adding the amount of daily energy accumulation in patients with malnutrition. Older underweight post-stroke patients in convalescent rehabilitation ward require about 9,600 kcal intake to gain 1 kg of body weight [90]. On the other hand, the amount of energy intake is set lower than their actual activity to lose weight in patients with obesity.
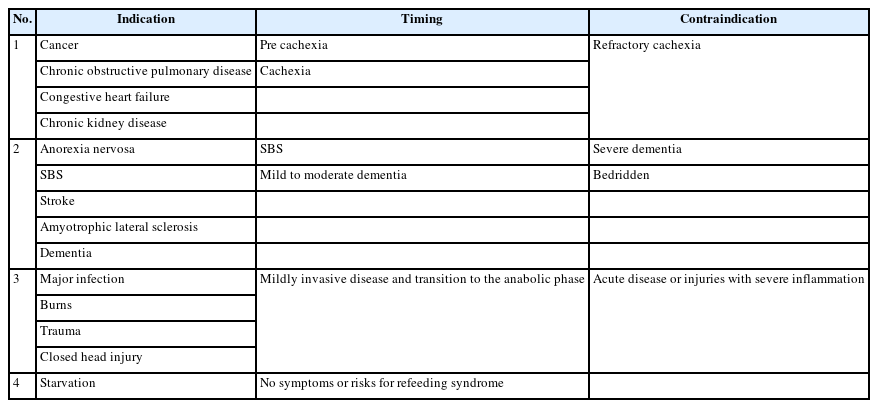
The indication and timing of aggressive nutrition therapy depends on the etiology and severity of malnutrition. The efficacy of therapy, side effects, and comorbidities are monitored during the therapy [35]. Aggressive nutritional therapy is planned and implemented based on the four etiologies of the Global Leadership Initiative on Malnutrition criteria (Table 1) [91]. Nutritional intake should be adjusted according to the activity level to prevent malnutrition and maintain physical function and muscle strength in patients with diabetes mellitus. Energy intake and protein are calculated based on kilograms of ideal body weight. Specifically, ≥25–35 kcal/kg of energy intake is recommended according to the level of physical activity, and ≥1.0 g/kg of protein is required per day [66]. Monitoring is necessary during aggressive nutrition therapy because side effects such as hyperglycemia, dyslipidemia, elevated blood urea nitrogen, liver dysfunction, and electrolyte abnormalities may occur. The frequency of monitoring should be weekly rather than monthly for patients in convalescent rehabilitation wards [35]. Determination of whether to continue or modify aggressive nutrition therapy based on monitoring of function, body weight, fat mass, and muscle mass.
Nutritional physical therapy
Nutritional physical therapy was defined by the Nutrition and Swallowing Physical Therapy Committee of the Japanese Physical Therapy Association in September 2017. Nutritional physical therapy is the practice of setting goals based on an understanding of malnutrition, sarcopenia, and excess or deficient nutritional intake to maximize an individual’s function, activity, participation, and quality of life. Therefore, physical therapists should share nutrition and physical therapy assessments with registered dietitians and other multidisciplinary professionals to determine nutritional care management and physical therapy that takes into account activity level, muscle tone, and involuntary movements [30].
Nutritional physical therapy overlaps with rehabilitation nutrition, and the two concepts share goals, assessment, and intervention strategies. The practice of these two concepts requires both perspectives [30]: physical therapy that considers nutrition status and nutritional care management that maximizes the effectiveness of physical therapy. Exercise therapy consists of flexibility exercises, resistance training, and aerobic training. Flexibility exercises are prescribed for patients with any nutritional condition. Patients with nutritional deficiencies should receive low-intensity exercise to maintain physical function. In contrast, patients with adequate nutrition should receive moderate or higher intensity exercise to improve physical function. Exercise load settings are determined using subjective exercise intensity (Borg scale) and other factors, and exercise frequency, intensity, and duration are adjusted. Exercise and nutrition therapy are effective, however few reports exist on the effectiveness of nutritional physiotherapy [30]. In addition, systematic clinical practice based on a bi-directional nutrition and physical therapy perspective is limited. Further clinical practice and studies of nutritional physical therapy are needed.
CONCLUSION
Sarcopenic dysphagia reduces swallowing function, ADL and life prognosis. The triad of rehabilitation, nutrition, and oral management may be useful for patients with dysphagia [92]. The triad of rehabilitation, nutrition and oral management requires a multimodal approach based on collaboration among different professions, institutions, and communities. Prevention of whole-body sarcopenia is the priority for sarcopenic dysphagia, and early diagnosis and treatment of sarcopenic dysphagia are essential. However, the use of tools diagnose sarcopenic dysphagia is inconsistent and heterogeneous, and validation against objective measures of swallowing dysfunction is limited [93]. A review of diagnostic criteria and the development of clinical practice guidelines for sarcopenic dysphagia are needed. The development of evidence on the simplified rehabilitation nutrition care process is required.
Notes
No potential conflict of interest relevant to this article was reported.
None.
Conceptualization: Wakabayashi H. Methodology: Wakabayashi H, Kakehi S. Formal analysis: all authors. Project administration: Wakabayashi H. Kakehi S. Visualization: Wakabayashi H, Ninomiya J, Shioya M. Writing – original draft: all authors. Writing – review and editing: Wakabayashi H, Kakehi S, Isono E. Approval of final manuscript: all authors.