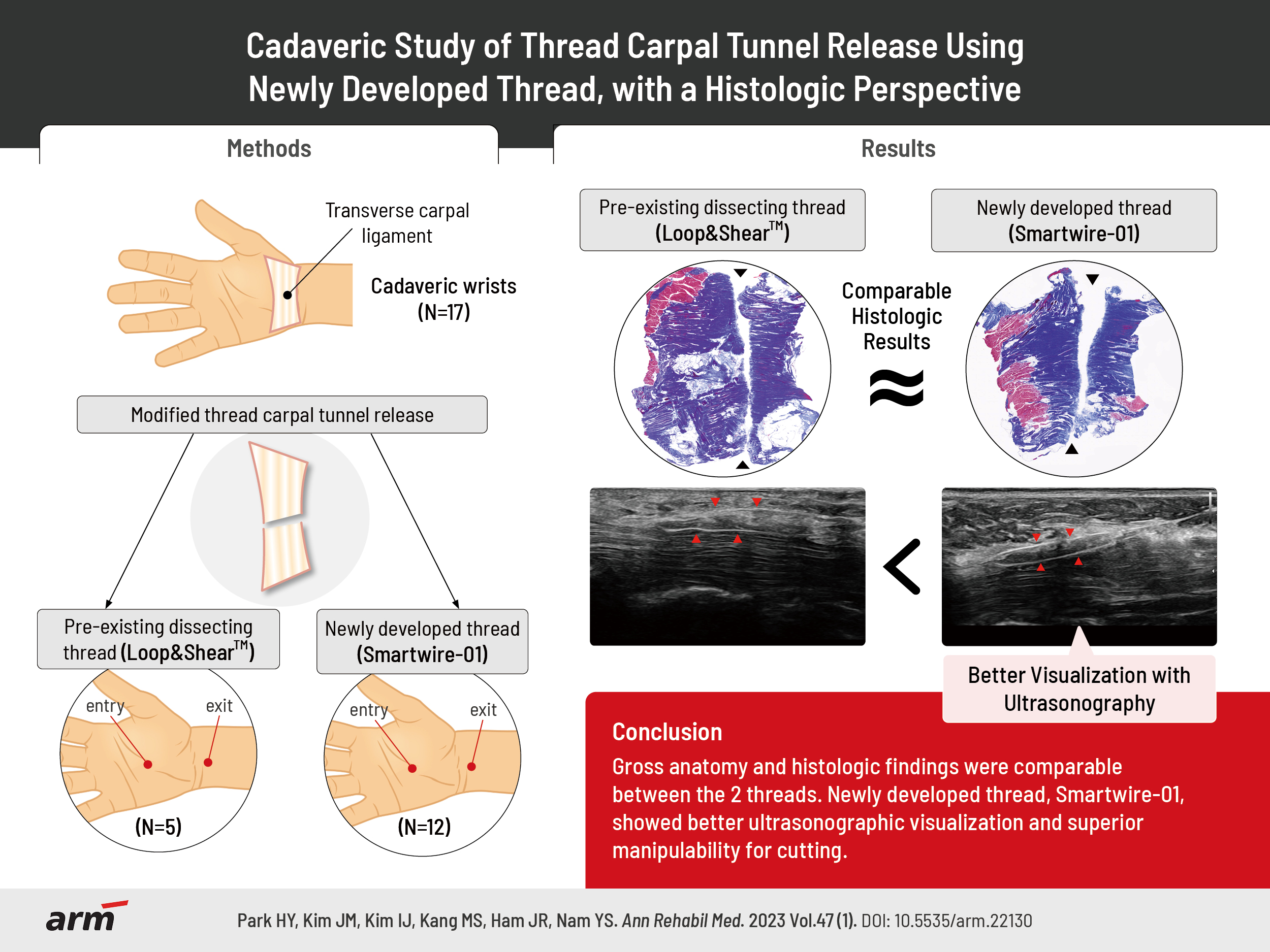
Cadaveric Study of Thread Carpal Tunnel Release Using Newly Developed Thread, With a Histologic Perspective
Article information
Abstract
Objective
To examine the usefulness and feasibility of modified thread carpal tunnel release (TCTR) by comparing the results of using pre-existing commercial thread with those of a newly developed thread (Smartwire-01).
Methods
A total of 17 cadaveric wrists were used in the study. The modified TCTR method was practiced by two different experts. Pre-existing commercial surgical dissecting thread (Loop&ShearTM) was used for five wrists and the newly developed Smartwire-01 was used for twelve wrists. The gross and microanatomy of the specimens were evaluated by a blinded anatomist.
Results
Both types of thread were able to cut the TCL similarly. Gross anatomy and histologic findings showed that there was no significant difference between the two types of threads. However, the practitioners felt that it was easier to cut the TCL using the newly-developed thread.
Conclusion
TCTR using Smartwire-01 was as effective as pre-existing Loop&ShearTM, with better user experiences.
INTRODUCTION
Carpal tunnel syndrome (CTS) is one of the most common entrapment neuropathies [1,2]. The carpal tunnel is a canal surrounded by bones and ligaments, and it contains flexor tendons and the median nerve. CTS occurs when the median nerve passing through the carpal tunnel is compressed due to various reasons. Compression of the median nerve at the wrist causes the clinical features of CTS, which include sensory disturbances in the first three digits, nocturnal paresthesia, and thenar muscle atrophy [2].
Both non-surgical and surgical interventions are considered in CTS treatment. Non-surgical treatment is commonly considered for the first-line management of CTS and includes oral steroids, splinting, ultrasound, and local corticosteroid injections [3-7]. The surgical treatment of CTS involves the transection of the transverse carpal ligament (TCL) and can be achieved by various methods. The traditional open technique involves a wrist skin incision, which may be associated with postoperative scars, pain, or infection. In contrast, the endoscopic technique is a minimally invasive approach associated with less scar pain and shorter recovery time [2,8]. However, the endoscopic technique has the disadvantage of high cost and is reported to show higher transient nerve injury [2,9]. Recently, Guo et al. [10] investigated a new minimally invasive CTS surgical treatment using ultrasound and thread. This technique, called thread carpal tunnel release (TCTR), accomplishes TCL transection by ultrasound guidance using a needle and dissecting thread, and leaves only needle insertion and exit sites after the procedure [10].
A previous study of modified TCTR with commercial dissecting thread (Loop&ShearTM; Ridge & Crest Company, Monterey Park, CA, USA) concluded that modified TCTR was safe and effective in treating CTS [11]. Recently, a novel dissecting thread was developed in Korea (Smartwire-01; Smart Wire Co., Ltd., Ilsan, Korea). However, the feasibility of using this newly developed thread in CTS has not been studied yet. Therefore, this cadaveric study was designed to investigate the usefulness of modified TCTR using the newly developed thread and compare the effectiveness and histologic findings with those of pre-existing commercial thread.
MATERIALS AND METHODS
Materials
Cadaveric wrists with no evidence of previous surgery or trauma history were included in the study. A total of nine fresh cadavers with 18 cadaveric wrists (four males and five females) were screened for the study, and one wrist was excluded due to a previous history of carpal tunnel release. The mean age of the cadavers was 78.7±8.7 years (male, 74.3±7.8; female, 82.2±8.5). The cadaveric specimens were obtained through the university-affiliated institute for applied anatomy, from February 2020 to January 2021. This study received an exempt determination from the Institutional Review Board of Catholic University of Korea because no personally identifiable information was used (no. CR-08).
Two types of threads were used in the study, pre-existing commercial dissecting thread (Loop&ShearTM) and the newly developed thread (Smartwire-01) (Fig. 1). Loop&ShearTM was used for three cadavers (five wrists) and Smartwire-01 was used for six cadavers (twelve wrists). Compared to the pre-existing dissecting thread (Loop&ShearTM, 0.23 mm in diameter, maximum load of 31.5 N), the newly developed Smartwire-01 was designed to have a larger diameter with a heavier maximum load (0.27 mm in diameter, maximum load of 42.4 N) for achieving a higher dissecting force. In addition, Smartwire-01 is coated with titanium nitride.
Modified thread carpal tunnel release
Prior to the intervention, the carpal tunnel structure was assessed by ultrasound (RS85; Samsung Medison, Seoul, Korea) with an L3-12A linear probe. After confirming that there were no structural abnormalities, a modified TCTR was performed. The ultrasound-guided modified TCTR method was performed by two different experts with over ten years of experience in ultrasound-guided interventions. The cadaveric wrists were randomly assigned to two different specialists at a 1:1 ratio using a block randomization procedure (block size of two). Because one wrist was excluded, each specialist performed ultrasound-guided modified TCTR procedures on eight and nine different cadaveric wrists, respectively.
The modified TCTR procedure was carried out similarly to that in a previous study by Guo et al. [12]. First, ultrasound was used in the hydrodissection of the median nerve at the carpal tunnel. An 18-G Tuohy needle attached to a syringe containing 5 mL of normal saline entered the palm, proximal to the superficial palmar arterial arch. With ultrasound guidance and simultaneous hydrodissection of the median nerve, the needle was advanced to the dorsal side of the TCL. After passing through the dorsal side of the TCL, the needle tip eventually exited at the wrist, proximal to the TCL. Then, the dissecting thread was inserted through the needle, and the needle was removed, leaving the thread on the dorsal side of the TCL. The second entry point of the needle was at the palm where the thread was located. Similar to the first entry, the needle passed through the palmar side of the TCL, eventually exiting at the same point as the first needle insertion, proximal to the TCL. Then, the tip of the pre-inserted thread was inserted through the needle. The dissecting thread was wrapped around the TCL after removing the needle, and by moving the thread reciprocally, the TCL dissection was completed.
Analysis
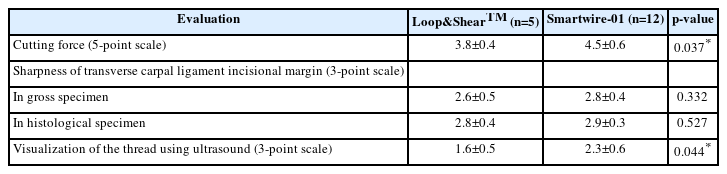
During the procedure, each practitioner evaluated the cutting force of the dissecting thread on a 5-point Likert scale. The cutting force of the Smartwire-01 was compared to that of the Loop&ShearTM, and the operators’ opinions were measured on a scale from 1 to 5, where 1 was very difficult to cut, 2 was difficult to cut, 3 was average, 4 was easily cut, and 5 was very easily cut.
Every procedure using an ultrasound probe was recorded, and an independent researcher who did not participate in the procedure evaluated the visualization of the thread using the recorded file. The researcher was blinded to the type of thread and assessed the visualization of the thread on a 3-point scale where 1 was not well visible, 2 was visible, and 3 was well visible. The blinded researcher also evaluated the incisional margin seen on ultrasound.
After the procedure, a blinded anatomist evaluated the precision of the TCL transection and any damage to the nearby structures in each cadaver through further dissection. TCL dissection was analyzed by dividing the procedures into “unable to cut,” and “able to cut.” The assessed structures included the TCL, the Berrettini branch, the digital nerve branch, and the median nerve. Gross structure evaluation included the sharpness of the incisional margins of the dissected TCL, where sharpness was evaluated on a 3-point scale where 1 was dull, 2 was neutral, and 3 was sharp.
Along with the evaluation of gross anatomy, histological analysis was also performed by the blinded anatomist. TCL tissue was sliced coronally using a rotary microtome (RM2255; Leica, Germany). To distinguish the collagenous fibers of the dissected TCL, Masson’s trichrome stain was used. The microscopic analysis included the shape and sharpness of the cut surface and any foreign material left after the dissection. The sharpness of the histological specimens was also evaluated on a 3-point scale.
Mann–Whitney U-test was performed in each evaluation for comparisons between Loop&ShearTM and Smartwire-01 thread. Statistical analyses were performed using IBM SPSS Statistics software (ver. 24.0; IBM Corp., Armonk, NY, USA).
RESULTS
Gross anatomy findings
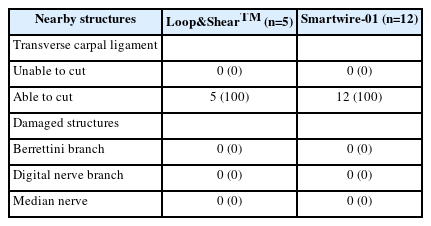
The evaluation of TCL dissection completeness by gross anatomy showed that both the Loop&ShearTM and Smartwire-01 threads were able to cut the TCL in all specimens (Table 1). Any damage to the nearby structures was also evaluated. Neither type of thread injured the Berrettini branch, the digital nerve branch, or the median nerve (Table 1).
The gross findings of the dissected TCLs are shown in Fig. 2. There were no significant differences between the Loop&ShearTM and Smartwire-01 thread groups in the sharpness of the incisional margin (3-point scale evaluation: Loop&ShearTM, 2.6 and Smartwire-01, 2.8; Table 2) or other anatomical differences.
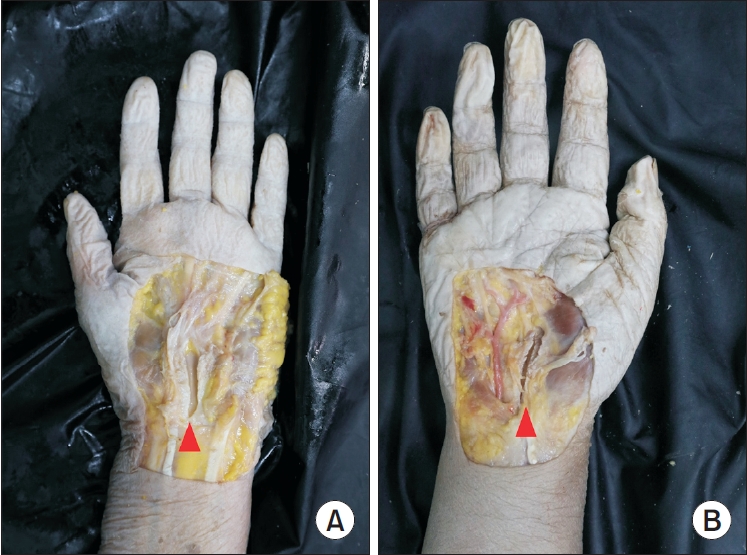
Gross findings of transverse carpal tunnel transection after modified thread carpal tunnel release. The red arrowheads indicate the transected transverse carpal ligament. (A) Dissection with Loop&ShearTM (Ridge & Crest Company, Monterey Park, CA, USA). (B) Dissection with Smartwire-01 (Smart Wire Co., Ltd., Ilsan, Korea).
Histological findings
The histological findings of the dissected TCL tissue are presented in Fig. 3. The cut surface of the TCL tissue, which is seen in blue color in Fig. 3, was similar when cut with either Loop&ShearTM or Smartwire-01. Sharpness evaluated on a 3-point scale showed no significant difference between the two threads (Loop&ShearTM, 2.8 and Smartwire-01, 2.9; Table 2). The incisional border of both types of thread was sharp and not irregular. In addition, no foreign substances were observed to be left at the incision site when evaluated under a microscope.
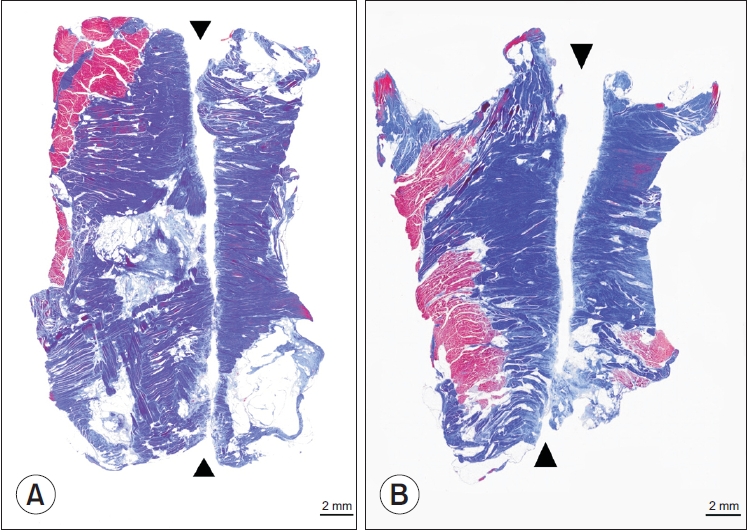
Histologic findings of the transverse carpal ligament (Masson’s trichrome stain, ×75). The black arrowheads indicate the transected transverse carpal ligament. (A) Dissection with Loop&ShearTM (Ridge & Crest Company, Monterey Park, CA, USA). (B) Dissection with Smartwire-01 (Smart Wire Co., Ltd., Ilsan, Korea).
Difference between threads during the procedure
During the procedure, the cutting force of the newly developed thread was evaluated as better than that of Loop&ShearTM (Loop&ShearTM, 3.8 and Smartwire-01, 4.5), meaning that the TCL was more easily cut using Smartwire-01 (Table 2). The cutting force did not differ between the two practitioners (practitioner 1, 4.5 and practitioner 2, 4.7). Compared to the Loop&ShearTM, Smartwire-01 was evaluated as being observed better by ultrasound during the procedure (3-point scale evaluation: Loop&ShearTM, 1.6 and Smartwire-01, 2.3; Table 2, Fig. 4). After the dissection, the incisional margin shown on ultrasound was similar between the two types of threads (Fig. 5).
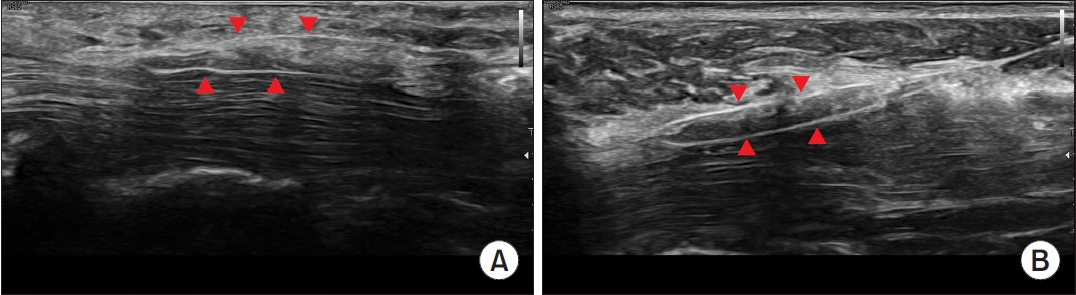
Visualization of the two threads on ultrasound. The red arrowheads indicate the looped thread surrounding the transverse carpal ligament. (A) Dissection with Loop&ShearTM (Ridge & Crest Company, Monterey Park, CA, USA). (B) Dissection with Smartwire-01 (Smart Wire Co., Ltd., Ilsan, Korea).
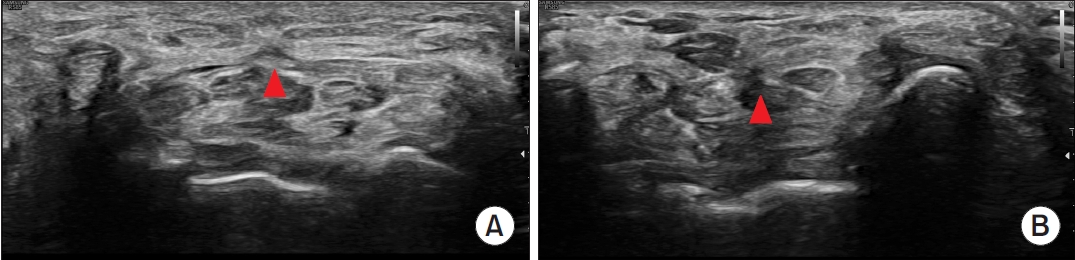
Dissected transverse carpal ligament shown on ultrasound. The red arrowheads indicate the transected transverse carpal ligament in the short-axis view. (A) Dissection with Loop&ShearTM (Ridge & Crest Company, Monterey Park, CA, USA). (B) Dissection with Smartwire-01 (Smart Wire Co., Ltd., Ilsan, Korea).
DISCUSSION
In this study, modified TCTR using the newly developed dissecting thread was as effective as the preexisting dissecting thread. Both threads were able to cut the TCL effectively, and there was no damage to the median nerve, Berrettini branch, or digital nerve branch after modified TCTR using either thread. Moreover, the sharpness of the incisional border in the histologic evaluation was similar in both groups. The practitioner felt higher cutting force with less resistance when using the newly developed thread, and the novel thread was visualized better on the ultrasound images.
Although the two thread types share similar chemical compositions, the maximum load, diameter, and coating material differ, and this may have resulted in the subjective differences observed during the procedure. When performing a modified TCTR, it is important to have a good visualization of the dissecting thread by ultrasound. The dissecting thread must be properly positioned so that the thread can wrap around the TCL. After clear visualization of the thread, the operator should not apply too much force when reciprocating the dissecting thread to prevent adjacent structural damage. In this study, the practitioners felt more comfort and less resistance during the dissection with Smartwire-01, which may have been due to the slightly larger diameter and higher maximum load of the newly-developed thread. In addition, Smartwire-01 was better visualized by ultrasound, which may have been due to the different coating (titanium nitride) used for Smartwire-01. Less resistance when cutting and better visualization in ultrasound support the feasibility of using Smartwire-01.
Patients with CTS often present with persistent, recurrent, or new symptoms following carpal tunnel release by the traditional open technique of carpal tunnel release [13]. The most common cause is the incomplete release of the TCL, and the second common is circumferential fibrosis around the median nerve [13]. Approximately 2%–10% of patients are reported to experience recurrent CTS, and efforts have been made to reduce the incidence of recurrent CTS [14,15].
The prognosis of patients undergoing modified TCTR has not been well reported yet. The Loop&ShearTM and Smarwire-01 threads used in this study showed clearcut surfaces in both gross and histologic findings. While open surgery involves the incision of nearby structures in order to dissect the TCL, modified TCTR only dissects the TCL, leaving a thread insertion/exit site. Since modified TCTR only cuts the target structure (TCL) and the cut surface is sharp, a lower risk of adhesion is expected, although prospective follow-up studies in clinical settings are needed. Moreover, no residual foreign material was seen in the histologic findings after modified TCTR using Smartwire-01 in this study. This suggests that the newly developed thread is safe to utilize in clinical settings.
Although Smartwire-01 was as effective as the pre-existing dissecting thread with better subjective user experiences, there were some limitations to the study. Due to the ultrasound guidance technique, the outcomes of the procedure may be affected by the practitioner’s ability to use ultrasound. Although there was no difference in outcomes between the two practitioners, the results may have differed based on the practitioner’s expertise. Also, although the practitioner’s cutting force scale may have been affected by the thickness of the TCL, we could not evaluate the association between TCL thickness and cutting force in this study. In addition, the Likert scale used in this study is a subjective measurement that has not been validated in other studies. In future research, it would be better to evaluate the newly-developed thread more objectively. Furthermore, because this study was a cadaveric study, the results cannot be generalized to real-world clinical settings. With the feasibility and advantages of Smartwire-01 found in this study, future studies should evaluate the effectiveness of Smartwire-01 in real-world clinical settings.
In conclusion, the results of modified TCTR using the Smartwire-01 thread were similar to the results of TCTR using Loop&ShearTM thread. Smartwire-01 was as effective as Loop&ShearTM and it was better visualized on ultrasound, with a better user experience. To our knowledge, this was the first cadaveric study to examine the effectiveness and microscopic structures of the newly developed thread in modified TCTR procedures. Further evaluation in clinical settings is needed.
Notes
Jung Ryul Ham reports non-financial support from Ultra V Co., Ltd. during the conduct of the study. Otherwise, no potential conflict of interest relevant to this article was reported.
Conceptualization: Kim JM, Kim IJ, Nam YS. Formal analysis: Nam YS. Funding acquisition: Kim JM. Investigation: Kim JM, Kim IJ, Park HY, Kang M. Methodology: Kim JM, Kim IJ, Ham JR, Nam YS. Writing - original draft: Park HY, Kim JM. Writing - review and editing: Kim IJ, Kang M, Ham JR, Nam YS. Approval of the final manuscript: all authors.
Acknowledgements
The new dissecting thread was made by Ultra V Co., Ltd. (#804, ShinHan IT Tower, 19 Sangwon-gil, Seongdong-gu, Seoul, Korea) and Smart Wire Co., Ltd. (#703, Moogoongwha-ro, Ilsan, Gyeonggi-do, Korea).
The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year 2020.
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2020R1F1A1055076).