Ultrasonographic Assessment of the Safe Zone for Carpal Tunnel Intervention: A Comparison Between Healthy Individuals and Patients With Carpal Tunnel Syndrome
Article information
Abstract
Objective
To compare transverse and longitudinal safe zones using ultrasonography between healthy individuals and patients with carpal tunnel syndrome (CTS).
Methods
This was a prospective observational case-control study. Forty wrists from 20 healthy individuals and 40 wrists from 24 patients with CTS were examined. Patients with CTS were classified into three groups (mild, moderate, and severe CTS) based on electrodiagnostic findings. Using ultrasonography, we measured the distance between the median nerve and ulnar vessels to identify the transverse safe zone, and between the distal flexor retinaculum and superficial palmar artery arch to identify the longitudinal safe zone.
Results
The transverse and longitudinal safe zones were significantly different between participants with CTS and those without CTS. The transverse safe zone significantly differed between the mild and severe CTS groups, while the longitudinal safe zone was not significantly different between the groups. The cross-sectional area of the median nerve negatively correlated with the transverse and longitudinal safe zones.
Conclusion
Transverse and longitudinal safe zones were narrower in patients with CTS than in the healthy group. A significant difference was observed between patients with mild CTS and those with severe CTS. Furthermore, the cross-sectional area of the median nerve was directly proportional to the degree of narrowing of the transverse and longitudinal safe zones.
INTRODUCTION
The carpal tunnel is a fibro-osseous tunnel in the wrist through which the flexor tendons and median nerve pass into the hand [1]. Carpal tunnel syndrome (CTS) is a clinical disorder caused by increased pressure on the median nerve within the carpal tunnel [2] and is the most common peripheral nerve entrapment neuropathy [3,4]
Several treatment options are available for CTS, and the preferred treatment depends on disease severity, symptom duration, and patient preferences [5]. If nonoperative methods, including wrist splinting, physical therapy, and corticosteroid injection, are ineffective, a surgical release should be considered. However, several studies have shown some degree of patient dissatisfaction after surgery owing to non-significant improvement in symptoms and significant postoperative complications, such as easy hand fatigability, decreased grip strength, incisional pain, and prolonged convalescence time [6,7].
Ultrasound-guided corticosteroid injections are as effective as surgery for the management of CTS [8,9]. However, their effectiveness is limited to mild CTS, and recurrence is a major concern.
Ultrasound-guided carpal tunnel release is a recently introduced technique for the management of CTS [10,11]. The procedure is performed as follows: under local anesthesia, a hook knife or needle is inserted parallel and medial to the median nerve under continuous sonographic guidance. The inserted hook knife or needle is advanced toward the distal flexor retinaculum but remains proximal to the superficial palmar arch artery. A complete release of the flexor retinaculum is then attempted, with care taken to prevent injury to the nerve or surrounding structures.
McShane et al. [11] reported a significantly reduced cross-sectional area (CSA) of the median nerve and improved functional outcomes after percutaneous needle tenotomy of the transverse ligament in patients with CTS. Moreover, Chern et al. [10] showed in a cadaveric study that most of the flexor retinaculum was completely released using an ultrasound-guided hook knife. RojoManaute et al. [12] showed that ultrasound-guided carpal tunnel release provided earlier functional improvement and less postoperative morbidity with the same neurological recovery as open carpal tunnel release in patients with symptomatic primary CTS.
In previous studies, the safe zone of the median nerve in the carpal tunnel has been defined and measured using several methods. Except for cadaveric studies, only two studies by Nakamichi and Tachibana [13] and Buncke et al. [14] have been conducted using ultrasound on living individuals. However, to the best of our knowledge, no sonographic study has investigated the longitudinal safe zone in living individuals and compared the safe zone of healthy individuals with that of patients with CTS.
This study aimed to identify the transverse and longitudinal safe zones of the median nerve in the carpal tunnel using ultrasonography in healthy individuals and patients with CTS of different severity. We hypothesized that the safe zone was narrower in patients with CTS than in healthy individuals, and the degree of narrowing was proportional to the severity of CTS.
MATERIALS AND METHODS
Participants
This prospective observational study was conducted at the Korea University Guro Hospital. We analyzed 40 wrists from 20 healthy participants and 40 wrists from 24 patients with CTS. CTS was diagnosed according to the American Association of Electrodiagnostic Medicine (AAEM) criteria, which include clinical history, symptoms, and electrophysiological evidence of slowing of the distal median nerve conduction [15].
The exclusion criteria were as follows: (1) prior surgery or trauma to the wrist or median nerve; (2) previous corticosteroid injections for CTS; (3) electrophysiological evidence that could mimic CTS or confuse its validation; and (4) history of diabetes mellitus, thyroid disease, or chronic kidney disease.
The study was approved by the Institutional Review Board of Korea University Guro Hospital (No. 2016GR0781). Informed consent was obtained from all the participants. Data on baseline characteristics, including age, sex, height, weight, arm length, and body mass index, were collected.
Nerve conduction studies
All participants underwent nerve conduction studies according to the AAEM protocol [15] before the ultrasound examination. The skin temperature was maintained at approximately 34°C during all the tests. Sensory nerve action potentials (SNAPs) were obtained antidromically using surface electrodes placed over the third digit. Compound muscle action potentials (CMAPs) were recorded from the abductor pollicis brevis muscle using surface electrodes in a belly-tendon montage.
The standard tests included median sensory nerve conduction velocity between the third digit and wrist segments and median distal motor latency from the wrist to the thenar eminence. If the results of standard tests were normal, further segmental tests were performed over a short distance of 7 cm, or comparative median/ulnar studies were performed. The reference values used in our study were as follows: (1) median nerve distal sensory latency, upper limit of normal 3.7 ms; (2) difference between the median and ulnar nerve distal sensory latencies, upper limit of normal 0.4 ms; (3) distal motor latency over the thenar eminence, upper limit of normal 4.2 ms; (4) median motor nerve conduction velocity, lower limit of normal 49 m/s; and (5) median sensory nerve conduction velocity, lower limit of normal 49 m/s [10]. The electrophysiological severity of CTS was classified into three groups according to the classification reported by Lee and Kwon [16] as follows: (1) mild group, abnormal sensory and distal motor latency; (2) moderate group, features of mild CTS with low SNAP amplitude (6–15 µV) and CMAP amplitude (2.1–4 mV); and (3) severe group, features of moderate CTS with very low SNAP amplitude (<5 µV) and CMAP amplitude (<2.0 mV) and fibrillations with abnormal abductor pollicis brevis motor unit action potentials on needle examination.
Ultrasound examinations
A 7–12-MHz linear-array transducer was used for all ultrasound examinations. Ultrasound measurements were performed by a physiatrist who was blinded to the electrodiagnostic (EDX) findings. The participants were seated on a chair with their forearm supinated and wrist extended at 20°, and the structures in the carpal tunnel were scanned. The proximal flexor retinaculum was identified based on the pisiform and scaphoid as medial and lateral landmarks, respectively. The distal flexor retinaculum at the level of the hamate was identified based on the following landmarks: the hook of the hamate medially and the trapezium laterally. Using a transverse section of the distal flexor retinaculum, we defined the transverse safe zone (TSZ) as the distance between the medial margin of the median nerve and the lateral margin of the ulnar vessels (Fig. 1A), while the longitudinal safe zone (LSZ) was defined as the distance between the distal end of the flexor retinaculum and the proximal end of the superficial palmar arch artery (Fig. 1B). The flexor retinaculum was echogenic and thickest at the distal portion of the longitudinal axis of the capitate bone and the proximal part of the third metacarpal bone.

Ultrasonographic measurement of (A) the transverse safe zone as the distance between the medial margin of the median nerve and lateral margin of the ulnar vessels; (B) the longitudinal safe zone as the distance between the distal end of the flexor retinaculum and proximal end of the superficial palmar arch artery.
The TSZ, LSZ, and CSA of the median nerve were measured thrice by a physician with 10 years of experience. For CSA measurement, the inner margin of the hyperechoic rim around the median nerve was traced. The average values were used for the analysis.
Statistical analysis
Statistical analysis was performed using SPSS Statistical Software for Windows version 22.0 (IBM SPSS, Armonk, New York, USA), and statistical significance was set at p<0.05. Data are presented as the mean±standard deviation. An independent t-test was used to compare the differences between healthy participants and patients with CTS. Analysis of variance (ANOVA) was performed to compare the safe zones among the mild, moderate, and severe CTS groups. Pearson correlation coefficient was used to determine associations between variables.
RESULTS
No significant differences were observed in the baseline characteristics of healthy participants and patients with CTS (Table 1).
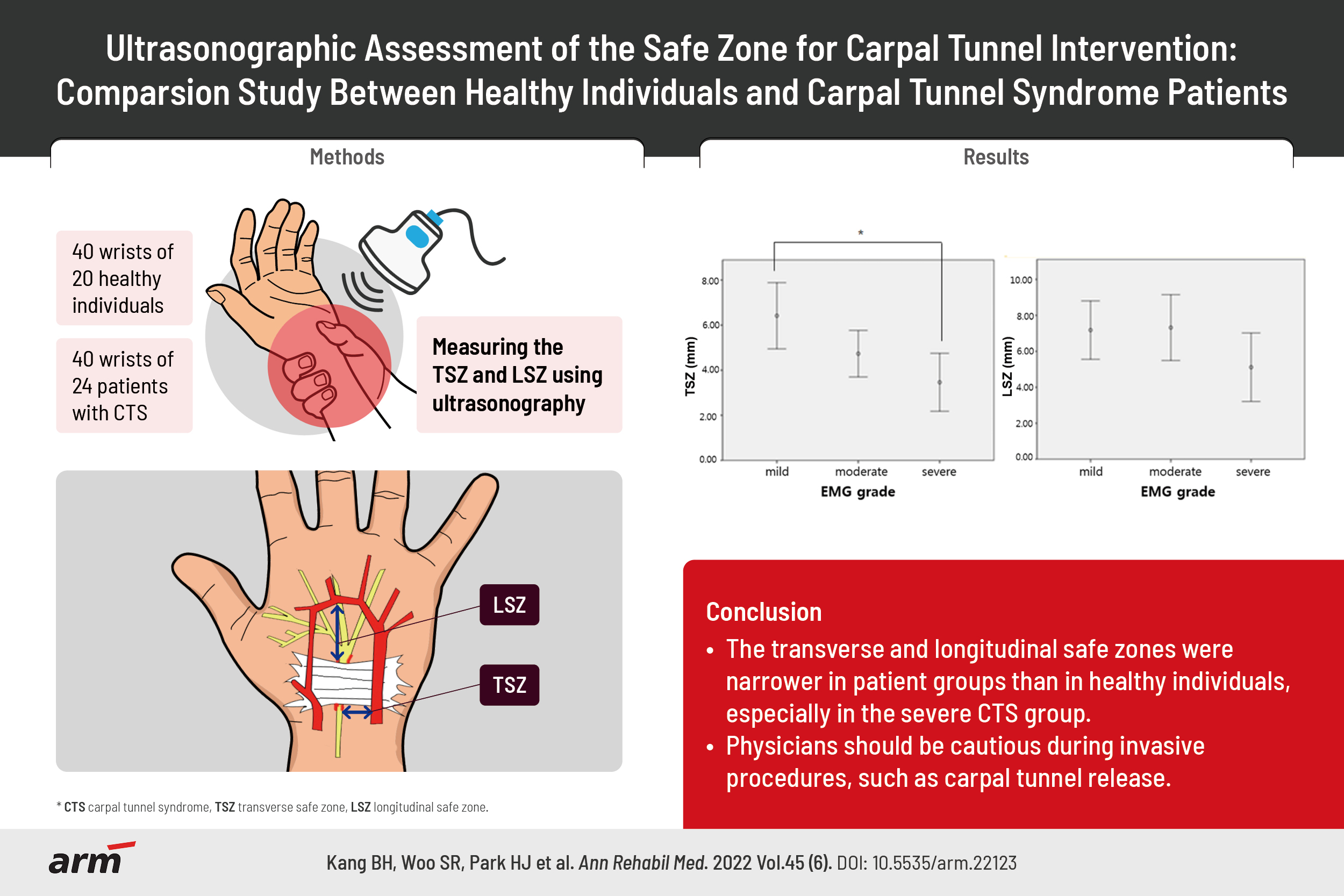
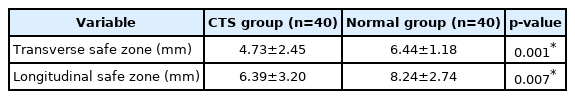
The mean TSZ was 6.44±1.18 mm in the healthy group and 4.73±2.45 mm in the CTS group. The mean LSZ was 8.24±2.74 mm in the healthy group and 6.39±3.20 mm in the CTS group. These results show that the TSZ and LSZ were significantly different between healthy participants and patients with CTS (p=0.001 and p=0.007, respectively) (Table 2).
Subgroup analyses using ANOVA based on EDX findings showed a significant difference in TSZ (p<0.05) between the mild and severe CTS groups. However, no significant difference in TSZ was observed between the mild and moderate CTS groups or between the moderate and severe CTS groups. We observed no significant intergroup differences in LSZ (Fig. 2).

Comparison of safe zones based on the severity of electromyographic (EMG) findings: (A) transverse safe zone (TSZ) and (B) longitudinal safe zone (LSZ). *p<0.05 using the Bonferroni test.
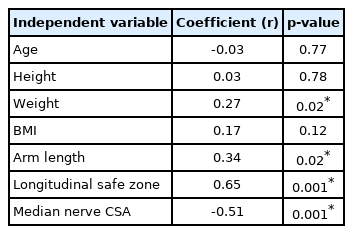
Multiple linear regression analysis was performed to determine the variables that were correlated with each safe zone. The results for TSZ, presented in Table 3, showed that four variables were significant predictors: weight, arm length, LSZ, and the CSA of the median nerve. Among them, the CSA of the median nerve showed a negative correlation, with a coefficient value of -0.51 (p=0.001). LSZ showed a positive correlation, with a coefficient value of 0.65 (p=0.001). The results for LSZ, presented in Table 4, showed that three variables were significant predictors: age, TSZ, and the CSA of the median nerve. Among them, the CSA of the median nerve showed a negative correlation, with a coefficient value of -0.37 (p=0.001).
DISCUSSION
The safety of minimally invasive carpal tunnel intervention has been extensively studied. In particular, the location of the inserted instrument is an important safety factor for minimally invasive carpal tunnel release under ultrasonographic or endoscopic guidance. In previous studies, the safe zone of the median nerve in the carpal tunnel has been described using various methods. Ajayi et al. [17] investigated the relationship between the radial styloid process and the ulnar styloid process with the median nerve in the carpal tunnel. They reported that the mean distances of the median nerve from the radial and ulnar styloid processes were 22.44 and 26.66 mm, respectively. Omokawa et al. [18] investigated the anatomical course of the ulnar artery and its branches towards the transverse carpal ligament and the location of the median nerve in 24 fresh cadaveric wrists. The mean distance between the distal margin of the transverse carpal ligament and the superficial palmar arch along the ring finger was 12 mm (range, 4–18 mm). The ulnar artery coursed from 7 mm ulnar to 2 mm radial to the hamate hook. The distance from the hamate hook to the median nerve was 11 mm. They reported that releasing the transverse carpal ligament 5 mm lateral to the radial margin of the hamate hook can prevent postoperative bleeding and avoid unexpected vascular and neural injuries. Nakamichi and Tachibana [13] measured TSZ in 60 wrists in 54 patients with CTS. They showed that the width of the TSZ at the distal wrist crease was significantly larger than that at any other wrist site. They also reported that the TSZ varied considerably among individuals. A few studies have measured the TSZ of the median nerve in patients with CTS. However, to the best of our knowledge, our study is the first in vivo study to additionally analyze the LSZ in participants with CTS and those without CTS. Moreover, this study is the first to compare the TSZ and LSZ according to the presence or severity of CTS.
In our study, the TSZ measured 4.73±2.45 mm and the LSZ measured 6.39±3.20 mm in patients with CTS. Chern et al. [10] reported a TSZ of 4.9 mm (range, 3–7 mm) and an LSZ of 11.5 mm (range, 6–16 mm) using ultrasound in cadaveric wrists. They used cadavers without a history of wrist or hand surgery; however, they did not report any history of CTS. Therefore, they did not determine the effect of CTS on the size of safe zones. The mean value of the LSZ measured in the study by Chern et al. [10] was shorter than that measured in our study. However, the range of the LSZ measured in the study by Chern et al. [10] mostly overlapped with the range measured in our study. Rotman and Manske [19] dissected 28 cadaveric hands to define the relationship between the median nerve and adjacent soft tissue structures. The distance from the distal edge of the transverse carpal ligament to the superficial palmar artery arch was measured in the sagittal section. The mean distance was 4.8±0.8 mm to the ring-finger axis and 5.5±0.7 mm to the long-ring-finger interspace axis. Olave et al. [20] dissected 56 cadaveric hands and reported that the mean LSZ measured 7.3±4.3 mm on the right and 8.3±3.5 mm on the left. The differences between the sexes and sides were not statistically significant.
However, previous cadaveric studies have yielded inconsistent results. As our study included a living, healthy population, we could determine the safe zone during carpal tunnel interventions, such as ultrasound-guided steroid injection or carpal tunnel release.
Compared to healthy participants, patients with CTS had narrower TSZ and LSZ. The narrower TSZ may be explained by median nerve swelling. We hypothesized that bowing of the flexor retinaculum, followed by median nerve swelling, might explain the narrower LSZ in patients with CTS. A three-dimensional approach is further required to verify this. Bowing of the flexor retinaculum affects the transverse as well as, the longitudinal sections. Based on our hypothesis, we examined 10 patients with CTS and median nerve swelling and observed that bowing of the flexor retinaculum resulted in narrow LSZ.
Regarding the differences in the safe zone according to the severity of CTS, a significant difference in TSZ was observed between the mild and severe CTS groups. According to the results of the previous studies, the CSA of the median nerve was associated with CTS severity [21-23]. However, in our study, no significant difference in TSZ was observed between the mild and moderate or the moderate and severe groups. Median nerve swelling is believed to be the most prominent feature of severe CTS. Therefore, practitioners should provide greater care to treat patients with severe CTS. In our study, we observed no significant difference in LSZ between the CTS groups. We assumed that bowing of the flexor retinaculum in addition to median nerve swelling resulted in a narrower LSZ. In our study, patients with CTS may not have had a sufficiently long disease course to cause flexor retinaculum bowing. Further studies that include patients with CTS with longer disease duration and more severe symptoms are needed to clarify this association.
In the multiple regression analysis, the CSA of the median nerve showed a negative correlation with the TSZ and LSZ. The significantly narrower TSZ and LSZ in patients with a larger CSA of the median nerve might be due to swelling of the median nerve and bowing of the flexor retinaculum. Moreover, when performing procedures in patients with a larger CSA of the median nerve, greater attention is needed.
As a significant difference was observed in TSZ and LSZ between patients with CTS and healthy participants, our study is meaningful in providing values that can improve the safety of carpal tunnel release, with or without ultrasonographic guidance. These findings suggest that imaging modalities, such as ultrasonography, should be used in procedures performed on patients with severe CTS. In a clinical setting, some situations might occur where physicians must perform a procedure using a blind technique. Blind carpal tunnel steroid injection is usually performed at the proximal carpal tunnel at the level of the distal wrist crease just ulnar to the palmaris longus tendon [24]. This is a potential space that indicates the TSZ in our study. These findings suggest that caution should be exercised when inserting the needle so that it is not located on the ulnar side in patients with severe CTS.
In endoscopic carpal tunnel release operations, the hook of the hamate is usually used as an important surface landmark. It is located 1 cm distal and radial to the pisiform bone. Kaplan’s cardinal line connecting the apex of the first web around the thumb with the hook of the hamate is used as the estimated distal end of the transverse carpal ligament [25]. The area distal to Kaplan’s cardinal line corresponds to the LSZ in our study, and this is the exit zone of the trocar in this operation. These findings might provide useful guidance for the detection of related structures, such as the superficial palmar arch or recurrent branch of the median nerve during endoscopic carpal tunnel release in patients with CTS.
This study had several limitations. The number of participants was relatively small; therefore, the statistical significance of the subgroup analysis based on CTS severity may be insufficient. In addition, because we did not perform an EDX study on the asymptomatic healthy participants, subclinical CTS could not be ruled out, though the possibility is low.
In conclusion, the TSZ and LSZ were narrower in patients with CTS than in healthy participants. In addition, the TSZ was significantly narrower in the severe CTS group than in the mild CTS group. Narrower TSZ and LSZ may be related to a larger CSA of the median nerve. These findings may provide useful information for safer, minimally invasive procedures for CTS, such as ultrasound-guided injection or carpal tunnel release.
Notes
No potential conflict of interest relevant to this article was reported.
Conceptualization: Kang BH, Woo SR, Park HJ, Chung SY, Kang S, Yoon JS. Methodology: Kang BH, Woo SR, Chung SY. Formal analysis: Kang BH, Woo SR, Chung SY, Kang S. Funding acquisition: Yoon JS. Project administration: Chung SY. Visualization:Kang BH, Woo SR, Chung SY, Kang S. Writing – original draft: Woo SR, Chung SY, Kang S, Yoon JS. Writing – review and editing: Kang BH, Woo SR, Park HJ. Approval of final manuscript: all authors.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (No. NRF-2020R1A2C1009024).