A New Ultrasound Method for Assessment of Head Shape Change in Infants With Plagiocephaly
Article information
Abstract
Objective
To compare a new ultrasound measurement method with calliper cephalometry in infants with deformational plagiocephaly (DP) and to assess the differences of two methods according to the severity of DP.
Methods
Fifty-two infants with DP were divided into two groups according to the degree of cranial vault asymmetry (CVA); group 1 included 42 infants with CVA over 10 mm, and group 2 included 10 infants with CVA under 10 mm. Cranial vault asymmetry index (CVAI) and occipital angle ratio (OAR) were measured by using calliper and ultrasound measurements, respectively. The occipital angle was defined as the angle between the lines projected along the lambdoid sutures of the skull.
Results
The occipital angles of the affected sides were significantly greater than those of unaffected sides in both groups. The CVAI and OAR were significantly greater in group 1 than in group 2 (CVAI, 9.3%±2.3% vs. 4.6%±1.5%; OAR, 1.05±0.4 vs. 1.01±0.0; p<0.05). The OAR was positively correlated with the CVAI in all infants (r=0.789) and in group 1 (r=0.784; p<0.05).
Conclusion
Our study revealed that OAR using the new ultrasound measurement was positively correlated with the CVAI in infants with DP. Therefore, the occipital angle measurement using ultrasound combined with cephalometry could provide better understanding about the characteristics of the overall cranial bone and lambdoid suture complex in infants with DP.
INTRODUCTION
Deformational plagiocephaly (DP), also known as 'positional plagiocephaly' or 'nonsynostotic plagiocephaly,' has become the most common infant craniofacial abnormality over the past decade [1]. The incidence of DP is estimated to be between 1/300 and 1/10 [1]. DP is a cranial asymmetry condition that presents as flattening of one side of the growing cranium, resulting from external forces during the prenatal, perinatal, or postnatal period [2,3,4].
Risk factors for DP include intrauterine constraint [5], assisted vaginal delivery, primiparity, prolonged labour [6], multiple births [7], male sex, unusual birth position [2], supine sleeping position [8], positional preference [9], and torticollis [10].
The diagnosis of DP is based on history, physical examination, calliper cephalometry, photography, and imaging studies including plain radiography and computed tomography (CT) [2,11,12,13,14]. Cephalometry is an inexpensive and non-invasive technique to measure skull asymmetry [15]. CT and ultrasound have been used as differential diagnostic methods for the evaluation of posterior plagiocephaly by assessing the appearance of the cranial sutures [16].
The concave side of the bone receiving non-physiological mechanical loading produces suppression of the growth at the growth plate [17]. Skull morphology is significantly influenced by the mechanical properties of osseous tissue. Previous studies [18,19,20,21,22] have found that new bone in the paediatric skull is produced at the sutural edges by proliferation of mesenchymal cells which differentiate into bone-forming osteoblasts, and that bone growth occurs preferentially at the suture interfaces according to the direction of the sutures.
As the periosteal tissues in skull expand with the growing brain, the bones of the skull suture move apart, and the mechanical loading strains the skull sutures to create the space and signal for the growth of a new bone. Therefore, the primary direction of bone growth in the paediatric skull tends to be toward the sutures, and the skull sutures determine the head shape [20].
The infant's skull has high flexibility for the brain growth. The average flexibility of the cranial bone with suture is 14%-40% higher than that of the cranial bone without suture, in both human and animal skulls [23,24]. Therefore, the suture of the infant skull is the most susceptible structure to the mechanical stress and deformation, and the skull shape is determined by the amount of two pressures in an opposite direction including internal distension pressure (brain growth) and external flattening pressure (compression by gravity during sleep) [25]. Measurement of suture direction may reflect the past or present mechanical force that has been applied to the skull.
Although cephalometry and radiography are helpful in rating the overall severity of plagiocephaly, they cannot measure the direction of the sutures and quantify deformities around the suture line. The purpose of this study was to develop an ultrasound-based method to measure the direction of the lambdoid sutures, to assess correlations between ultrasonographic measurements and cephalometry, and to evaluate any differences in correlations between ultrasound measurements and the severity of head deformity in infants with DP.
MATERIALS AND METHODS
This study was conducted at an outpatient clinic of the Department of Rehabilitation Medicine at Daegu Catholic University Hospital from 2012 to 2013. The study was performed after receiving approval from the Institutional Review Board and Ethics Committee at the Daegu Catholic University Hospital, in accordance with the Declaration of Helsinki.
Participants
Fifty-two infants visiting the outpatient clinic in the Department of Rehabilitation Medicine at Daegu Catholic University Hospital were recruited. The infants who met the following criteria were enrolled in this study: 1) a cranial asymmetry that presents as flattening of one side of the growing cranium and 2) the ability to comply with cephalometry and ultrasound measurements. The infants with craniosynostosis were excluded from this study.
Clinical measurements
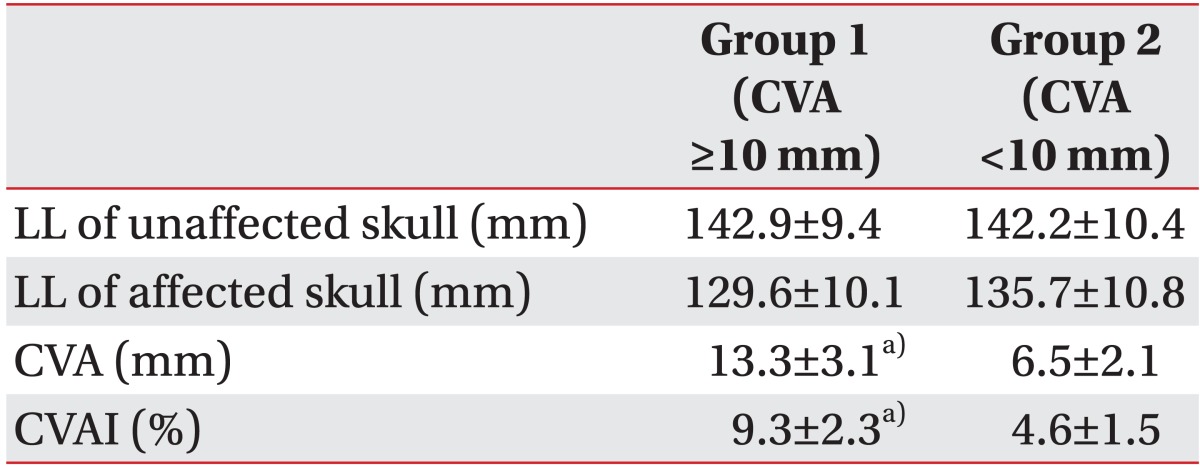
Calliper cephalometry was performed by a paediatric physiatrist. Lateral length was defined as the distance between the frontozygomatic and contralateral occipital bones and was measured on both the unaffected (a) and affected (b) sides of the skull (Fig. 1A). Cranial vault asymmetry (CVA) was calculated by subtracting the lateral length of the affected side (b) from that of the unaffected side (a). The cranial vault asymmetry index (CVAI) was calculated by dividing CVA (a-b) by the lateral length of the unaffected side (a) and multiplying by 100. The infants were divided into two groups according to CVA: 21 boys and 21 girls (mean age, 8.5±5.2 months) with over 10 mm of CVA were assigned to group 1, and 5 boys and 5 girls (mean age, 8.6±3.2 months) with under 10 mm of CVA were assigned to group 2.
Ultrasound measurements
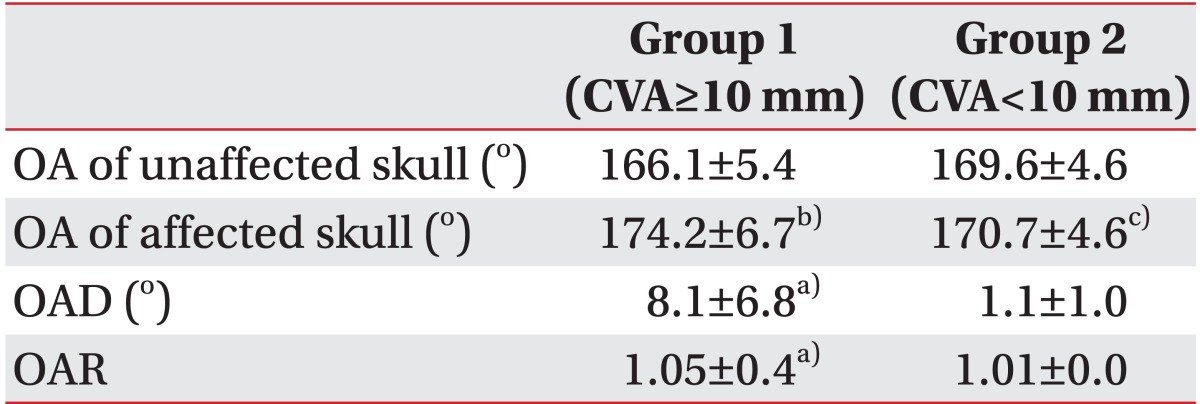
All ultrasound measurements were performed by a physiatrist with 10 years of experience in performing musculoskeletal ultrasound using an Antares (Siemens Healthcare, Erlangen, Germany) system with a 5-13 MHz multi-frequency linear transducer. All infants were scanned in the prone position on an examination table while asleep. Ultrasound was discontinued if the infant became tense and uncooperative. The entire skull was scanned from the posterior fontanelle to the mastoid fontanelle. The transducer was placed perpendicular to the lambdoid sutures, and transverse scanning of both sutures was performed. A short cine loop of the affected and unaffected occipital bones at their flattest parts was acquired. The occipital angle was defined as the angle between lines projecting along the lambdoid sutures of the skull (Fig. 1B, C). Two ultrasound measurements were obtained to check intra-rater reliability. The occipital angles of both the affected and unaffected sides were measured. The occipital angle ratio (OAR) was calculated as the mean occipital angle of the affected side divided by the angle of the unaffected side.

Cranial vault asymmetry index (CVAI) was calculated using caliper (A). The cranial diagonal diameter was measured on unaffected (a) and affected sides of skull (a>b). Cranial vault asymmetry (CVA) is defined as the difference between the cranial diagonal diameters (a-b) divided by long cranial diagonal diameter (a); and CVAI is CVA multiplied by 100. An occipital angle was measured on ultrasound image in unaffected (B) and affected skull (C). A straight line between the two end points was drawn along the calvaria on the left and right sides of the lambdoid suture (arrow). The occipital angle was defined as the angle between straight lines that was drawn from each end point of the lambdoid suture along the skull. The occipital angle was 167° at unaffected (B) and 184° at affected skull (C).
Statistical analysis
The statistical analysis was performed using SPSS ver. 14.0 software (IBM, Armonk, NY, USA) with the level of significance set at p<0.05. Data are presented as the mean±standard deviation. Intergroup differences with respect to measured parameters were analysed using the Mann-Whitney U test. The independent t-test was used to compare the occipital angles of the unaffected and affected sides in group 1, and Wilcoxon signed-rank test was used in group 2. Pearson correlation coefficient was used to analyse the correlation between CVAI and OAR. An interclass correlation coefficient was used to examine the intra-rater reliability of repeated occipital angle measurements.
RESULTS
Clinical and ultrasound measurements
The lateral lengths of the affected and unaffected sides were 129.6±10.1 mm and 142.9±9.4 mm in group 1 and 135.7±10.8 mm and 142.2±10.4 mm in group 2, respectively. Group 1 CVA (13.3±3.1 mm) and CVAI (9.3%±2.3%) values were significantly greater than group 2 CVA (6.5±2.1 mm) and CVAI (4.6%±1.5%) values (p<0.05) (Table 1).
The occipital angles of the affected and unaffected sides were 174.2°±6.7° and 166.1°±5.4° in group 1 and 170.7°±4.6° and 169.6°±4.6° in group 2, respectively; and the occipital angles of the affected sides were significantly greater than those of unaffected sides (Table 2). However, group 1 demonstrated an occipital angle difference (OAD) between the affected and unaffected sides of 8.1°±6.8° and an OAR of 1.05±0.4, which were significantly greater than the OAD (1.1°±1.0°) and OAR (1.01±0.0) values observed in Group 2 (Table 2).
Intra-rater reliability of repeated occipital angle measurements
The interclass correlation coefficients for repeated occipital angle measurements of the affected and unaffected sides were 0.915 and 0.926, respectively.
Correlation between CVAI and OAR
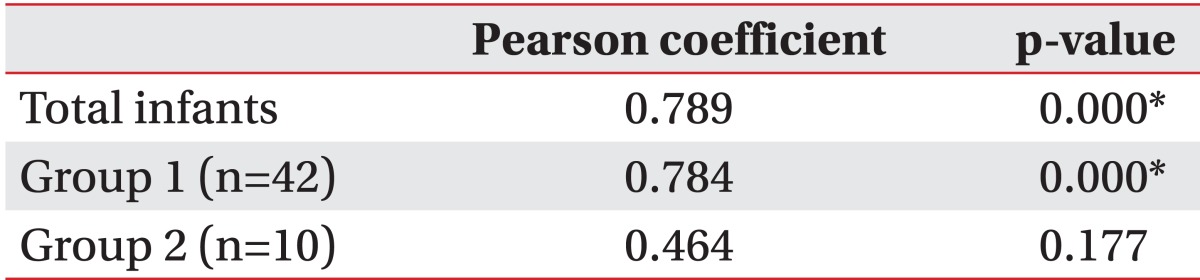
The OAR was positively correlated with the CVAI in all infants (r=0.789) and in group 1 (r=0.784; p<0.05). However, there was no significant correlation between CVAI and OAR in group 2 (r=0.464, p=0.177) (Table 3).
DISCUSSION
Our results showed that the occipital angle of the affected side found by using the new ultrasound measurement was significantly greater than those of the unaffected side in infants with DP. However, OAD and OAR of infants with CVA over 10 mm were significantly higher than those in infants with CVA under 10 mm. Since the primary direction of bone growth in an infant's skull is determined at the suture, the direction of the skull suture may be useful in predicting skull growth tendencies. To date, no study has described the direction of the lambdoid sutures using ultrasound in infants with DP. In previous studies, the use of ultrasound was limited to investigating the patency of the lambdoid sutures [16,26]. We developed an ultrasound measurement of the occipital angle to assess the direction of the lambdoid sutures of infants with DP. The occipital angle was defined as the angle between lines projecting along the lambdoid sutures of the skull. In our study, the occipital angle changed according to the direction of the lambdoid suture, which increased as the direction of the suture became flattening. The direction of skull sutures can be measured using CT. Previous studies [27,28] have shown that CT can provide quantitative measurements of skull ossification and bone configuration around the suture. However, CT examination involves a significant radiation dose [29,30,31]. In addition, the sedation necessary for CT imaging in infants is associated with risks. Ultrasound has advantages, such as having no radiation exposure or no sedation requirement. The direction of skull sutures can be clearly detected using ultrasound. In our study, infants with DP were classified into two groups based on a cutoff value of 10 mm of CVA. Although the application of an orthotic is usually recommended for the infants with severe DP, the criteria for sever DP is still being argued [32]. Mortenson and Steinbok [15] defined as severe the DP over 12 mm of CVA, and Rogers [33] recommended the orthotic therapy for DP over 10 mm of CVA. Yoo et al. [34] reported the clinical outcomes of cranial moulding therapy in infants with DP, and the infants were classified into two groups according to 10 mm of CVA. The therapeutic effect of cranial moulding therapy was shown to be less in infants with under 10 mm of CVA and greater in infants with over 10 mm of CVA. They strongly recommended the cranial moulding therapy to be used in infants with over 10 mm of CVA.
The CVAI was positively correlated with the OAR in all infants with over 10 mm of CVA. Cephalometry verified the degree of severity of DP [4]. Calliper cephalometry is a simple and non-invasive method of investigating the effect of cranial remodelling orthoses and providing precise information about the main diagnostic features in infants with DP [35]; it has high reproducibility and low intraobserver and interobserver variability [36]. Although it provides information about overall skull deformity, it cannot assess the morphology around the lambdoid sutures. To the best of our knowledge, our study is the first to evaluate the correlation between cephalometric and ultrasound measurements in infants with DP. Our results suggest that a clinical assessment using cephalometry with ultrasound may be useful to establish a treatment plan and to evaluate the effects of therapeutic modalities in infants with DP. In our study, infants with less than 10 mm of CVA did not show a correlation between CVAI and OAR. This may be due to the small number of subjects in this group.
The intra-rater reliability of occipital angle measurements using ultrasound was excellent. This result was in agreement with the previous study [26] investigating the patency of lambdoid sutures using ultrasound in infants with DP.
Our study has several limitations. First, a small number of infants was included, especially those with CVA under 10 mm. Second, we could not measure the occipital angle in normal infants. Third, we could not evaluate the interrater reliability of ultrasound measurements according to the experience of the examiner, because some parents did not adhere to the study protocol. Finally, follow-up ultrasound measurements were not performed after the treatment. Further studies with a larger sample size, follow-up ultrasound measurements after treatment, assessment of inter-rater reliability, and comparison of occipital angle between infants with and without DP are needed to confirm the validity of our results.
In conclusion, the occipital angle of the affected side obtained by using the new ultrasound measurement were significantly greater than that of the unaffected side, and the OCR was positively correlated with the CVAI in infants with DP. Since the occipital angle is related to the direction of the lambdoid suture, the occipital angle measurement using ultrasound combined with cephalometry could provide better understanding about the characteristics of the overall cranial bone and lambdoid suture complex in infants with DP.
Notes
No potential conflict of interest relevant to this article was reported.