Early Return to Play After Anterior Cruciate Ligament Reconstruction: Is It Worth the Risk?
Article information
Abstract
Objective
To compare the outcomes of a 6-month-long accelerated rehabilitation with a 12-month-long rehabilitation. There is no consensus on the optimal duration of rehabilitation after anterior cruciate ligament reconstruction (ACLR). Trends in the past decades have shifted towards accelerated programs, often resulting in a return to play (RTP) at 4–6 months, postoperatively. However, longer rehabilitation cycles have recently experienced renaissance due to a greater understanding of graft remodeling.
Methods
Adult athletes who underwent ACLR between 2015 and 2018 by the same surgeon were included and followed-up prospectively for 24 months. Participants were allocated into two groups based on their RTP (6 months vs. 12 months) and compared with graft elongation, reoperation rate, and sports career (quit or continue) outcomes.
Results
Fifty-four patients underwent accelerated rehabilitation and 92 completed conventional rehabilitation. The accelerated rehabilitation was significantly associated with graft elongation—the accelerated rehabilitation group (n=9) and the conventional rehabilitation group (n=0), p<0.001—and need for reoperation—the accelerated rehabilitation group (n=5) and the conventional rehabilitation group (n=1), p=0.026. Although the relationship between rehabilitation time and quitting competitive sports did not reach significance at 0.05 level (p=0.063), it was significant when p<0.1, thereby showing a clear trend.
Conclusion
Accelerated rehabilitation increased graft elongation risk. Knee laxity ≥3 mm measured at 6 months after ACLR should be accompanied by RTP time frame re-evaluation. Arthrometry checkups or routine magnetic resonance imaging shortly after RTP may be considered in cases of accelerated rehabilitation.
INTRODUCTION
Anterior cruciate ligament reconstruction (ACLR) with a bone-patellar tendon-bone (BPTB) graft is one of the most commonly performed procedures in sports medicine [1,2]. Despite its high success rate, the reported ipsilateral graft failure rate of 7%–9% is still a threat to the careers of athletes in cutting and pivoting sports [3,4]. Although there is no universally accepted definition of graft failure, the most common approach is based on objective and subjective signs of knee instability [5,6]. Graft rupture and elongation without rupture are common patterns that cause persistent subjective instability and increased objective knee laxity after an ACLR [6]. Several factors have been suggested to pose an increased risk of graft failure, including improper graft placement, inadequate fixation, tunnel malposition, use of allograft tissues, young age, and early return to play (RTP) [4,6]. The ACLR surgery is often accompanied by a loss of a full season; nevertheless, no consensus has been reached regarding the optimal duration of postoperative rehabilitation. Trends in the past decades have shifted towards accelerated programs, often resulting in an RTP between 4–6 months, postoperatively [5,7]; however, longer rehabilitation cycles have recently experienced renaissance due to a greater understanding of graft healing and restoration timelines [8]. Therefore, it is important to note that human anterior cruciate ligament (ACL) grafts undergo extensive biological remodeling and incorporation after implantation, and are still immature even at 1 year, postoperatively [9,10].
The present study shares our experience with a 6-month-long accelerated rehabilitation after ACLR surgery with a patellar tendon autograft. We hypothesized that RTP at 6 months postoperatively is associated with an increased risk of graft elongation without rupture. To test our hypothesis, we compared the functional results of athletes completing either accelerated (6 months), or conventional (12 months) rehabilitation after ACLR at our institute. Additionally, we aimed to identify additional risk factors for graft failure within the first year after surgery.
MATERIALS AND METHODS
Study design
A total of 146 consecutive patients were prospectively enrolled in the study between 2015 and 2018 at a single level I trauma center located in an urban area. Level I qualifications are based on national standards, regarding the types of resources available and number of patients admitted annually. The designation criteria corresponded to the standards of the United States of America [11,12]. The study complies with the Declaration of Helsinki and has been approved by the local medical ethics committee at the University of Szeged (Regional and Institutional Review Board of Human Investigations, Chairman: Prof. Dr. Tibor Wittmann) under reference number 10/2021- SZTE.
Inclusion criteria
Adult athletes (age, ≥18 years) who underwent ACLR surgery with a BPTB autograft by the same orthopedic trauma surgeon were included in our analysis. Patients who performed only recreational sports or were physically inactive were excluded.
ACLR surgery
The patellar graft was harvested from the central part of the tendon, and the position of the tunnel was anatomical in each case. The single-bundle technique was used. All the patients underwent preoperative rehabilitation, according to similar principles. In cases of concomitant injury, preoperative protocols were adjusted accordingly. Meniscus injuries entailed arthroscopic resection or reinsertion, and 6–16 weeks of recovery prior to ACLR surgery. Patients with concomitant medial collateral ligament (MCL) tears received functional braces and 8–12 weeks recovery period before the ACLR.
Patient groups
Participants were allocated into two groups based on their time frames for the RTP. Conventional and accelerated postoperative rehabilitation schedules were used for patients undergoing ACLR. Both programs were divided into six phases, which used the traffic light concept [13] at the end of each rehabilitation stage (Supplementary Table S1). However, the duration of phases 4–6 differed significantly between the two protocols. Consequently, the patients aimed to return to sport practices at either 12 or 6 months, postoperatively. Choosing the appropriate program was a shared decision between the patient and surgeon, and taking individual risk stratification into consideration. General health status and physical condition, concomitant injuries, compliance, and the explicit wishes of the patient were important factors in making the final decision.
Follow-up
A 24-month follow-up period was scheduled for each patient. Sagittal laxity of the operated knee was measured routinely with the KT-2000 arthrometer at 6 weeks, 3 months, 6 months, and 12 months after the surgery. After the 12 months, laxity was measured only in patients with subjective knee instability. Magnetic resonance imaging (MRI) was performed in cases of pain, instability, or increased knee laxity (6 mm or greater, or a difference of 3 mm or greater compared to the healthy side). At 24 months, all the patients were contacted and asked about their physical condition and whether they could compete at the same level as before their ACL injury.
Data collection
The recorded variables included age, sex, concomitant injuries, body mass index (BMI), comorbidities, practiced sport, early postoperative complications (such as hemarthrosis or infection), chronic postoperative complications (such as cyclops syndrome or graft elongation), injuries during the 24-month-long follow-up, sagittal laxity of the operated knee at 6 weeks and 3, 6, and 12 months after surgery, reoperation due to graft failure, and outcome with regards to sports career (quit or continue). Since joint hypermobility may influence the results of knee arthrometry, the Beighton score was recorded for each patient to assess the prevalence of generalized joint laxity (GJL) in the study population, which we defined as a Beighton score ≥5/9 based on the recommendation of the 2017 International Classification of the Ehlers-Danlos syndrome [14].
Outcomes
As a primary goal, conventional and accelerated rehabilitation groups were compared with respect to graft elongation, reoperation, and sports career outcomes (cessation or continuation). Graft elongation can be defined as an unstable knee with an unruptured ACL graft [3]. Although a difference of >3 mm in anteroposterior laxity compared with a healthy knee raises the possibility of elongation [6], a more informative assessment of the graft length and position can be obtained with an MRI [15]. In our study, elongation was defined as an increased graft length confirmed by MRI accompanied by subjective instability and increased laxity. As a secondary goal, we aimed to disclose associations between individual variables (age, sex, comorbidities, type of sport practiced, hemarthrosis in the early postoperative phase, meniscus injury during rehabilitation, cyclops syndrome, arthrometry results) and graft failure (rupture or elongation without rupture).
Statistical analysis
Continuous data were expressed as mean±standard deviation. Categorical data are expressed as frequencies or relative frequencies (percentages). The Fisher exact tests were performed to assess the relationship between postoperative rehabilitation and graft elongation, reoperation and quitting sporting careers within two years after the surgery. Because of the existing literature on age as a risk factor for graft failure [4,16,17], it was tested separately using a receiver operating characteristic (ROC) analysis. The area under the ROC curve (AUC) was calculated. The 95% confidence intervals (CI) for the AUC-ROC were calculated using the non-parametric method. To identify other risk factors for graft elongation, a multivariate logistic regression analysis was performed. The forward likelihood ratio model selection method was used. Statistical significance was set at p<0.05. Statistical analysis was performed using IBM SPSS version 24 statistical software (IBM, Armonk, NY, USA).
To investigate the statistical power of the applied tests, a post hoc power analysis was performed using the statistical software G*Power version 3.1.9.7 (Heinrich Heine Universität, Düsseldorf, Germany).
Efforts to reduce bias
Several measures were taken to reduce the bias. The patients were selected based on objective criteria—ACLR surgery with single-bundle BPTB method by the same surgeon (LT), practicing sports at a professional level. A suitable rehabilitation program was chosen based on a unified risk stratification protocol; however, patient
preferences also influenced this decision. Each participant completed the 24-month follow-up period. The validity of the statistical tests performed in our study setting was investigated using a post-hoc power analysis.
RESULTS
Patient population
A total of 326 patients underwent ACLR performed by the same orthopedic trauma surgeon in our institution between 2015 and 2018. Finally, 146 participants met the inclusion criteria. The decision for accelerated rehabilitation was made in 54 patients. A flowchart of patient enrolment is presented in Fig. 1.

Diagram showing patient enrolment. Between 2015 and 2018, 326 patients underwent ACLR performed by the same orthopedic trauma surgeon. The one-patellar tendon-bone technique was used in 169 patients. After excluding patients who did not compete in sports, 146 athletes were included in the analysis. The decision for accelerated rehabilitation was made in 54 cases, and the remaining 92 patients completed a 12-month-old recovery schedule. ACLR, anterior cruciate ligament reconstruction; BPTB, bone-patellar tendon-bone.
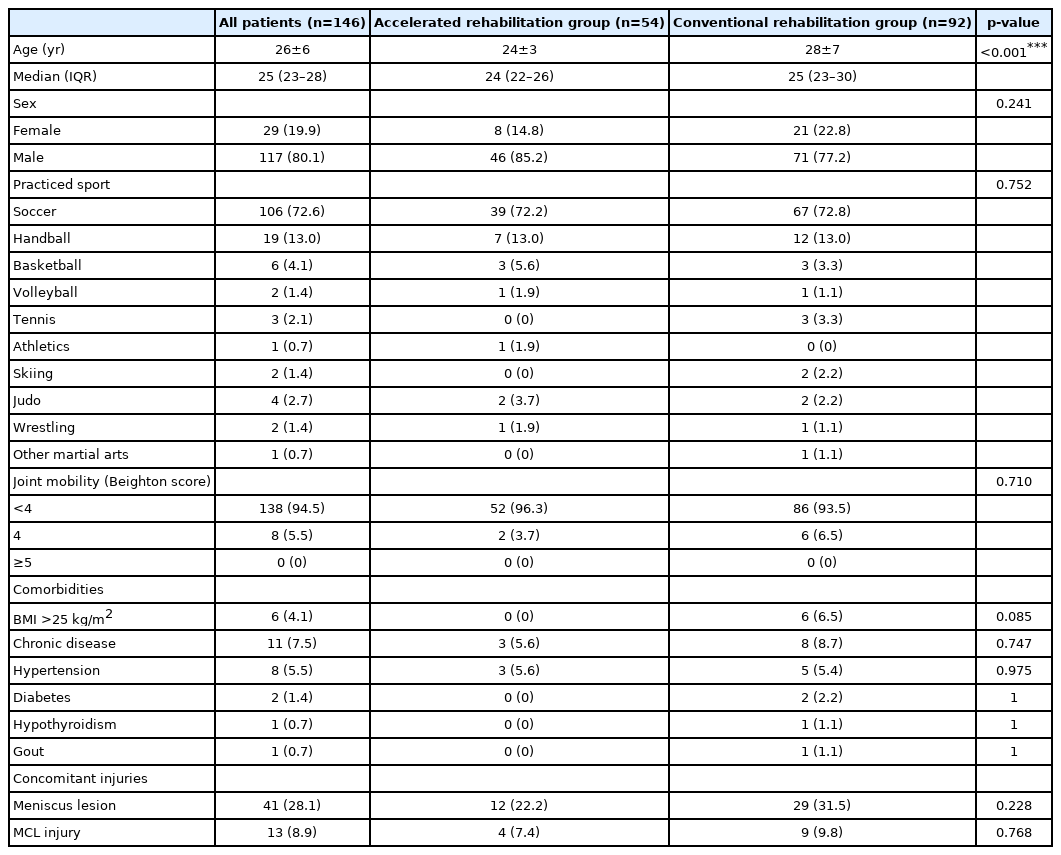
Patient characteristics
The mean age of the participants was 26±6 years, and only 19.9% were women. Soccer players were clearly overrepresented (72.6%) in the study population compared to other athletes. Only 4.1% of the patients had a BMI of over 25 kg/m2, 5.5% had hypertension, and 1.4% had diabetes (non-insulin dependent). None of the patients displayed GJL, while moderate hypermobility (Beighton score=4) was detected in 5.5% of the participants. The patient characteristics are shown in Table 1.
Postoperative complications and results of statistical analyses
Complications in the postoperative phase and longterm outcomes are summarized in Table 2. The primary outcomes are demonstrated in Fig. 2. All the patients complied with the guidance of their surgeons and regulations of the rehabilitation protocols.

Primary outcomes in the study groups. The distribution of graft elongation, reoperation, and quitting sports careers among the study groups are presented. Black indicates the conventional rehabilitation cohort, while white represents the accelerated rehabilitation group. Most importantly, graft elongation without rupture occurred only in the patients who completed the accelerated program. Furthermore, there was a notable difference in the reoperation rates and terminating sports careers between the study groups. *p=0.0261, ***p<0.001 (Fisher exact test).
It is important to emphasize that graft elongation occurred exclusively in the accelerated rehabilitation group, and a statistically significant relationship was confirmed (p<0.001). Accelerated rehabilitation also showed a significant relationship with reoperation due to graft failure (p=0.026). Although earlier RTP also entailed a higher rate for quitting competitive sports (7.4% vs. 1.1%), their statistical association did not reach significance at the 0.05 level (p=0.063); however, it would be significant in the case of p<0.1.
According to the literature, patient age is an important risk factor for graft failure [14,18,19]. In our patient population, most athletes were in their 20s, and age did not reach significance at the 0.05 level (AUC=0.669, p=0.075) in the ROC analysis (Fig. 3). Nonetheless, a p-value of 0.075 indicated a clear trend.
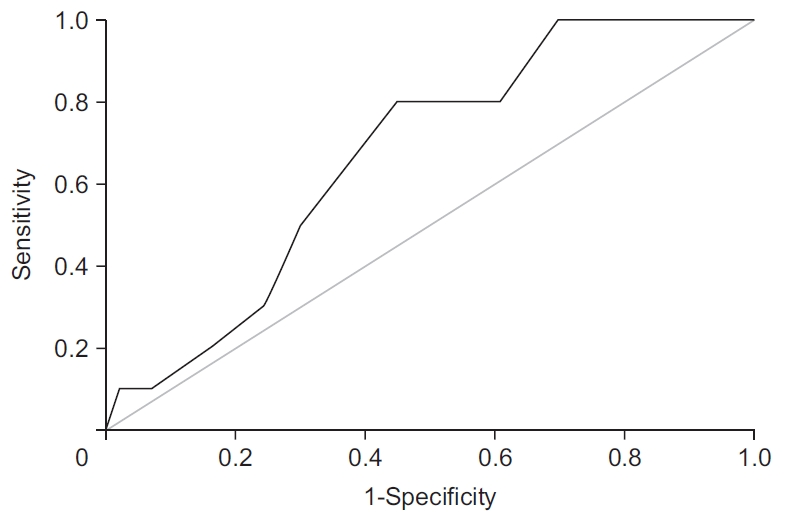
Relationship of patient age with graft failure. Receiver operating characteristic (ROC) curve demonstrated a relationship between age and graft failure. Despite being confirmed as a risk factor for graft failure in several studies, patient age was not significantly associated with graft failure in our patient population.
In addition to investigating accelerated rehabilitation, we aimed to identify other factors that carry the risk of graft failure. Multiple logistic regression was used for further analysis. The test revealed that slightly higher knee laxity (≥3 mm) measured with an arthrometer 6 months after ACLR was associated with graft failure during the subsequent 6 months—odds ratio [OR]=2.062; 95% CI, 1.019–4.172; p=0.044). Other variables (sex, BMI, type of sport practiced, hypertension, diabetes, hemarthrosis in the early postoperative phase, meniscus injury during rehabilitation, cyclops syndrome, and arthrometry results at 6 weeks and 3 months) could not be identified as risk factors.
The statistical power of the applied tests was investigated using a post-hoc power analysis. With a total sample size of 146, the graft failure rate of 9.3% in the accelerated rehabilitation group and 1.1% in the conventional rehabilitation (control) group provided more than 90% (92%) statistical power for the test of independence.
DISCUSSION
The present study compared the outcomes of 6- and 12-months-long rehabilitation programs after ACLR surgery in a level I trauma center. With regard to graft elongation and reoperation rates, longer rehabilitation resulted in significantly better results. Interestingly, the failure pattern of elongation without rupture occurred only in cases of return to play at 6 months, postoperatively.
The history of accelerated rehabilitation after ACLR started with the recognition of the outstanding functional results of noncompliant patients who avoided complications originating from postoperative immobilization. Since postoperative recovery traditionally began with 2 weeks of immobilization of the operated knee at 30°, and weight bearing was permitted only from the 8 week, patients suffered from flexion contracture, extensor mechanism dysfunction, and muscle atrophy [20]. Additionally, early mobilization reduces the risk of thromboembolism and fosters mental wellbeing. Thus, the traditional approach to rehabilitation has become outdated, and individualized, accelerated protocols allowing RTP already after 4–6 months came into practice [9,21]. However, there is no consensus regarding the optimal postoperative recovery program [22]. A prospective, randomized, double-blind comparison of accelerated and nonaccelerated rehabilitation programs found no difference in anterior knee laxity and functional performance during 2 years of follow-up; nevertheless, the value of this study is limited because of the small number of patients (n=24) [23]. Recent studies have raised concerns about early RTP, as accelerated rehabilitation programs have been associated with secondary ACL injury, increased knee laxity, proprioceptive deficits, and muscle strength imbalance (contralateral differences and antagonist/agonist ratio) [11,24-26]. A study of 234 athletes undergoing ACLR found that objective functional recovery (assessed with isokinetic and hop tests) of the knee was generally unsatisfactory at 6 months after ACLR [27]. Furthermore, a better understanding of graft remodeling does not support early RTP. Additionally, the tensile strength of the graft is only 65%–70% of the peak strength at 24 weeks after the surgery [19], and ultrastructural studies have shown that the mean fibril diameter of the graft is significantly different at 6 and 12 months, postoperatively [21]. The total collagen content and non-reducible/reducible crosslink ratio continued to increase in the graft during the first year [28]. These data suggest that athletes returning to competitive sports 6 months after ACLR, expose their insufficiently integrated graft to peak load. Although the ligamentization process takes several years [11], the first 12 months may be important for long-term functional outcomes.
The structural environment of the graft and appropriate mechanical stress, directly influence the remodeling processes, thereby referring to the adequacy of the surgical technique and rehabilitation regime [11]. Consequently, if technical failure, such as tunnel mal-positioning or incorrect fixation cannot be confirmed in cases of graft failure without trauma, the rehabilitation program is likely to be the main contributor to the unfavorable outcome. Accordingly, a significant association was found between RTP at 6 months and the presence of an elongated graft at 12 months in the present study. All the patients with graft elongation displayed a knee laxity ≥6 mm on arthrometry and a well-positioned but loosened graft on MRI. Although increased knee laxity and instability would indicate surgical management, only 62.5% of patients with elongated grafts agreed to reoperation. In the case of revision ACLR, we managed to keep the original graft in 71.4% of the patients and replaced it with the hamstring tendon in 29.6%. Of the reoperated athletes, 85.7% could continue competitive sports, demonstrating the high success rate of the revision ACLR. This may also explain why the association between accelerated rehabilitation and quitting sports careers did not reach the significance level in our study.
It is important to emphasize that the patients in the present study did not undergo traditional rehabilitation with excessive immobilization and prolonged nonweight bearing. As presented in Supplementary Table S1, both protocols are individualized and they aim for early restoration of full range of motion (ROM). The differences occur only in the later rehabilitation phases, where a longer schedule represents a more cautious approach to sport-specific drills, endurance training, and competitive situations in pivoting sports. Based on the experience of the past decade, our current practice favors RTP between 9–12 months in a majority of the patients.
In addition to the surgical techniques and postoperative rehabilitation regimes, other factors may be contributing to graft failure. According to a widely accepted theory, younger, more active patients tend to put more demand on the graft; thus, increasing the risk of re-injury and elongation [29]. Lower age has been confirmed as a risk factor in several studies [9,14,29,30]; however, it was not significant at the 0.05 level in our analysis. It should be noted that our patient population consisted mainly of young adult athletes; adolescents and amateur sportsmen were excluded. Therefore, a p-value of 0.075 implies that there may be an association between age and graft failure in more heterogeneous patient groups. Ultimately, clear conclusions about the effect of age on the outcome of ACLR in the general population should not be drawn purely on this study.
There is controversy in the literature regarding the influence of higher BMI (>25 kg/m2) on graft failure and the need for revision surgery [31-33]. Interestingly, individuals with BMI between 25–30 kg/m2 may be the most prone to graft failure [31]; consequently, a cut-off point of 25 was chosen to assess BMI as a risk factor in our study. Despite biomechanical differences in terms of muscle control in patients with different BMI values [34], the association of BMI >25 kg/m2 with graft failure could not be confirmed in our study. However, this result has limited value because the prevalence of BMI >25 kg/m2 was generally low in our patient population.
The role of sex in the long-term success rates of ACLR is also controversial. Although male sex was accompanied by a higher risk of revision surgery in some studies, there is no clear consensus [30,31]. Sex had no significant effect on graft function in our analysis.
According to our findings, arthrometry performed 6 months after ACLR may indicate an increased risk of graft failure during the subsequent six months. A knee laxity of 3 mm or greater should raise attention to the increased risk of graft elongation in cases of early RTP. Switching to a longer rehabilitation program or closer follow-up with or without routine MRI should be considered in such cases.
Our study had some limitations, most importantly, was the lack of random allocation. Since ACLR puts the future career of athletes on the line, we considered it more ethical to involve patients in decision-making.
The success of rehabilitation also depends on the psychological state of patients, which were not assessed comprehensively in this study. Although major compliance problems did not occur, personal differences in motivation levels and pain tolerance could potentially influence outcomes.
The ability to continue competitive sports after ACLR surgery is a success in itself. However, a more detailed survey, assessing finer changes in athletic performance has not been completed.
Our patient population displayed relative homogeneity in certain characteristics. Besides the low rate of ties, GJL (Beighton score ≥5) did not occur, and there were only a few cases of mild hypermobility comorbidi(Beighton score=4). Consequently, an adequate assessment of these parameters could not be performed.
The use of reoperation as an outcome does not reflect the actual failure rate after primary ACLR. Revision surgery was not performed in all the patients with insufficient functional results [33]. According to other studies, the actual failure rate can be twice as high as the number of revision surgeries [35].
In conclusion, early RTP after accelerated rehabilitation entails an increased risk of graft elongation without rupture. Knee laxity ≥3 mm measured 6 months after ACLR should be accompanied by RTP time frame reevaluation. Switching to a longer rehabilitation program or closer follow-up, with or without routine MRI, may be considered in such cases.
Notes
No potential conflict of interest relevant to this article was reported.
Conceptualization: Török L, Hartmann P, Jávor P. Formal analysis: Rárosi F. Funding Acquisition: Hartmann P, Jávor P. Project administration: Hartmann P. Visualization: Török K. Writing–original draft: Jávor P. Writing–review and editing: Török L, Hartmann P, Török K. Approval of the final manuscript: all authors.
Acknowledgements
The study was funded by a National Research Development and Innovation Office grant (FK138839). PH was further supported by a Bolyai János Grant from the Hungarian Science Academy (BO/00605/21/5). PJ was funded by a Human Resource Development Operational Programme Grant (EFOP-3.6.3-VEKOP-16-2017-00009). The funders did not influence the design or scientific content of our paper.
SUPPLEMENTARY MATERIALS
Supplementary Materials can be found via https://doi.org/10.5535/arm.22010.
Protocols for postoperative rehabilitation after ACLR surgery