Development of the Korea Dysarthria Test Following Stroke
Article information
Abstract
Objective
To develop an objective and quantitative clinical evaluation tool that can be used for diagnosis and severity assessment of dysarthria in patients with stroke.
Methods
A prototype test comprising 23 items was developed to test the function of each speech organ. The scoring of the prototype test was based on the analysis of the result values obtained from 50 healthy individuals. The test was performed for 50 patients with stroke who were suspected to have dysarthria. For evaluating the correlation between each prototype test item and the Urimal Test of Articulation and Phonation (U-TAP), the odds ratio was obtained for each result, based on which the final test items for composing the Korea Dysarthria Test (KDT) were selected. The validity of the test was evaluated using the receiver operator characteristic (ROC) curve and the area under the curve. We used the intraclass correlation coefficients to quantify inter- and intra-rater reliability. The Spearman correlation coefficient was used for examining the correlation between the KDT and the Speech Mechanism Screening Test and U-TAP.
Results
Among the 23 prototype test items, 16 exhibiting significant results were finally selected as the KDT. The higher score of the KDT is reflected the better speaking function. The sensitivity and specificity of the KDT were shown to be high at the cutoff value of 76.50 point.
Conclusion
KDT is a useful evaluation tool for dysarthria, showing a significant correlation with SMST and U-TAP.
INTRODUCTION
Dysarthria is one of the most common communication disorders following stroke besides aphasia—a disorder caused by a dysfunction in the central nervous, peripheral nervous, or musculoskeletal system [1,2]. For the detection of aphasia, various screening tests have been developed, such as the Frenchay Aphasia Screening Test [3], Bedside Evaluation Screening Test [4], Sheffield Screening Test for Acquired Language Disorders [5], Sklar Aphasia Scale [6], and Aphasia Language Performance Scales [7]. In South Korea, the Korean version of the Frenchay Aphasia Screening Test (K-FAST) has been developed and is widely used [8].
However, despite numerous reports regarding the highest prevalence of dysarthria among the neurogenic language disorders following stroke, research pertaining to the development of screening or diagnostic tests for dysarthria is scarce [1,2,9]. To date, studies have reported its occurrence owing to disturbed motor control of the facial muscles, jaw, tongue, lips, pharynx, and larynx, the consequence of which includes decreased speech rate, slow oral movement, reduced ability of resonance or vocalization, hypernasality, and distorted pronunciation [1,10]. Additionally, patients with dysarthria were reported to show ineffective control of various organs engaged in language production as well as respiration, vocalization, resonance, articulation, and prosody [11,12].
Based on these findings, several studies have attempted to develop a diagnostic test for dysarthria. Darly et al. [13] proposed a method of categorization according to auditory perception, which could not be widely used, as it was subjective and lacked inter-rater consistency [13-15]. Currently, the Frenchay dysarthria assessment developed by Enderby [16] is the most commonly used standard tool; however, the lack of a Korean version of this tool implies that there is no standard diagnostic test for dysarthria in South Korea [15-17], where the Urimal Test of Articulation and Phonation (U-TAP) is primarily used for the diagnosis of dysarthria. However, the U-TAP was limited to the assessment of consonant accuracy and focused more on the articulation test, while the studies on U-TAP targeted pediatric patients [18-20].
Additionally, the Speech Mechanism Screening Test (SMST) is currently the main screening test for dysarthria. Although the SMST is a screening test with high validity and reliability [21], it has a drawback of a relatively long testing time of 30–40 minutes. Additionally, whereas the target age of the participants in the SMST is 18–59 years [21], a stroke commonly occurs in adults aged ≥65 years, implicating a limitation of applying the SMST to such patients [22]. Therefore, a greater number of studies should be conducted for screening and diagnosis of patients with stroke-related dysarthria in South Korea.
Therefore, the present study aimed to develop a diagnostic tool for dysarthria with a focus on the early diagnosis and language rehabilitation for preventing patients with stroke from experiencing challenges in performing their roles as a member of the society. The study also aimed to develop an objective and quantitative test taking into account the correlation with the U-TAP, a widely used diagnostic test for dysarthria in South Korea, while designing the test with simple but comprehensive categories addressing the function of each speech organ. Accordingly, we developed a final test by selecting 16 items with high relevance to U-TAP from the prototype test consisting of 23 items. We named this diagnostic tool the Korea Dysarthria Test (KDT).
MATERIALS AND METHODS
Subjects
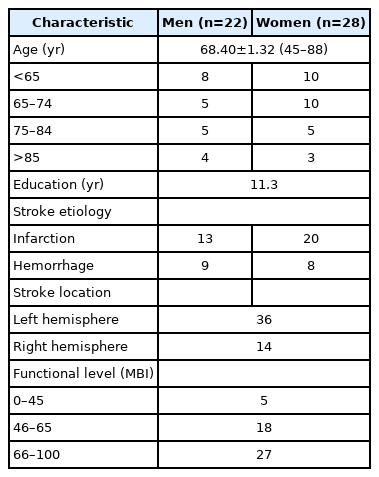
The participants in this study were 50 patients with stroke and suspected dysarthria, for whom the requested tests were performed by the Department of Rehabilitation at Chungbuk National University Hospital, during December 2018–March 2019. The average age of the participants was 68.40±1.32 years, with 22 men and 28 women (Table 1). The inclusion criteria were the Glasgow Coma Scale ≥14 point, age ≥18 years, the onset of stroke within 6 months, and individuals suspected of pure dys- arthria. In contrast, the exclusion criteria, were as follows: an uncooperative individual, an individual who could not maintain a seated position for 10 minutes or more, an individual with a history of the oropharynx or esophagus surgery such as tracheostomy, an individual with a problem of anatomy such as cleft lip or cleft palate, an individual with aphasia, apraxia of speech, oral apraxia, or fluency disorder, in addition to dysarthria, and an individual with severe visual or auditory impairment. For establishing the scoring system of the prototype test, the test was performed for 50 healthy individuals with no history or sign of dysarthria, while the average age of these individuals was 64.10±1.58 years, with 24 men and 26 women. Informed consent was obtained from all study participants. The study protocol was reviewed and approved by the Institutional Review Boards of Chungbuk National University Hospital (No. 2018-01-019).
Methods
The sample size in this study was estimated after assuming 10% drop-out, and based on this, 50 participants were recruited. The prototype test comprised the items that allowed the assessment of the functions of the lips, tongue, soft palate, and vocal cord that is related to speech, as well as respiration, and the ability of articulation and phonation. Additionally, some items allowed the assessment of the specific signs of dysarthria. The test was performed for 50 healthy individuals by a single speech therapist (Rater A). The results of the test were analyzed and each item was expressed as mean and standard deviation, while based on this, the scoring system was constructed for each item of the prototype test. Using the scoring system, the same speech therapist (Rater A) performed the test for 50 patients with stroke. For estimating the inter-rater reliability, a different speech therapist (Rater B) performed the retest at hourly intervals for 20 of the 50 patients. Later, a prospective U-TAP was performed for the patients by a rehabilitation medicine doctor (Rater C) for confirming dysarthria. For the assessment, each rater was prevented from knowing the test results from others. The protocol of Kim and Shin [18] was used for the U-TAP, which was applied equally to all patients.
Test protocol
To develop the prototype test, two rehabilitation medicine specialists compiled nearly all the previously developed tools for dysarthria [1,10,18,19,21,23-26] and reorganized the items to allow objective evaluation. The prototype test comprised the items focusing on the strength and control of each organ and the coordination ability. The items were thus grouped into six categories: lip, tongue, soft palate, vocal cord, respiration, and articulation and phonation, which were then further divided among 23 sub-categories. An overview of the study design and process is given in Fig. 1.

An overview of the study design and process. U-TAP, Urimal Test of Articulation and Phonation; KDT, Korea Dysarthria Test; OR, odds ratio.
Lip
The lip function was broadly categorized into the symmetry, strength, and control of the lip. The symmetry of the lip was subjectively assessed by the rater based on five stages—normal (100% left-right symmetry), slightly asymmetrical (≥75% and <100%), asymmetrical (≥50% and <75%), severely asymmetrical (≥25% and <50%), and extremely severe asymmetry (<25%). The strength of the lip was assessed using two items. First, while the patient was holding a tongue depressor with his or her lips, the rater tried to pull out the device with force, and the intensity of the force the patient applied to the lips was subjectively assessed based on five stages—normal, slightly decreased, decreased, severely decreased, and extremely severe decrease. Second, the patient was instructed to repeat the bilabial /bbʌ/ (/뻐/) sound as loud as possible, and the intensity was subjectively assessed based on five stages—normal, slightly decreased, decreased, severely decreased, and extremely severe decrease. The control of lip was assessed using the repeated /pʌ/ (/퍼/) and /Ɔi/ (/ 오이/) sounds. The patient was instructed to pronounce the bilabial /pʌ/ (/퍼/) sound for 5 seconds quickly, and the number of repetitions was measured, based on five stages—normal (≥20 times), slightly decreased (≥17 and ≤19 times), decreased (≥14 and ≤16 times), severely decreased (≥11 and ≤13 times), and extremely severe decrease (10 times). The patient was then instructed to pronounce the /Ɔi/ (/오이/) sound for 5 seconds quickly, and the number of repetitions was measured, which was assessed based on five stages—normal (≥11 times), slightly decreased (≥9 and ≤10 times), decreased (≥7 and ≤8 times), severely decreased (≥5 and ≤6 times), and extremely severe decrease (≤4 times).
Tongue
The tongue function was broadly categorized into the strength and control of the tongue. The strength of the tongue was assessed using the following items: tongue tip elevation, /ddʌ/ (/떠/) sound, and /kkʌ/ (/꺼/) sound. For tongue tip elevation, the rater pushed the tongue depressor on the patient’s tongue and the intensity of the force the patient applied in lifting the device with his or her tongue was subjectively assessed based on five stages—normal (elevation against strong resistance), slightly decreased (elevation against weak resistance), decreased (complete elevation in absence of resistance), severely decreased (slight elevation in absence of resistance), and extremely severe decrease (no elevation even in absence of resistance). For /ddʌ/ (/떠/) and /kkʌ/ (/ 꺼/) sounds, the patient was guided to pronounce /ddʌ/ (/ 떠/) and /kkʌ/ (/꺼/) sounds, five times each and as loud as possible, then the intensity was assessed based on the five stages—normal, slightly decreased, decreased, severely decreased, and extremely severe decrease. The control of the tongue was assessed using the following items: /tʌ/ (/터/) repeat, /rʌ/ (/러/) repeat, and /kʌ/ (/ 커/) repeat. For /tʌ/ (/터/) repeat, the patient was guided to pronounce the alveolar (plosive) /tʌ/ (/터/) sound for 5 seconds promptly, and the number of repetitions was measured, based on the five stages—normal (≥19 times), slightly decreased (≥16 and ≤ 18 times), decreased (≥13 and ≤15 times), severely decreased (≥10 and ≤12 times), and extremely severe decrease (≤9 times). For /rʌ/ (/ 러/) repeat, the patient was guided to pronounce the alveolar (liquid) /rʌ/ (/러/) sound for 5 seconds promptly, and the number of repetitions was measured, based on five stages—normal (≥18 times), slightly decreased (≥15 and ≤17 times), decreased (≥12 and ≤14 times), severely decreased (≥9 and ≤11 times), and extremely severe decrease (≤8 times). For /kʌ/ (/커/) repeat, the patient was guided to pronounce the velar /kʌ/ (/커/) sound for 5 seconds promptly, and the number of repetitions was measured, based on five stages—normal (≥18 times), slightly decreased (≥15 and ≤17 times), decreased (≥12 and ≤14 times), severely decreased (≥9 and ≤11 times), and extremely severe decrease (≤8 times).
Soft palate
The soft palate function was assessed by monitoring the movement of the soft palate and the level of nasal sound released upon making an oral sound. For the movement of the soft palate, the patient was instructed to pronounce /α/ (/아/) while the rater gently pushed the patient’s tongue with a depressor, and the elevation and symmetry of the soft palate were subjectively assessed based on five stages—normal (symmetrical and normal elevation), slightly decreased (slightly asymmetrical or slightly reduced elevation), decreased (asymmetrical or reduced elevation), severely decreased (severely asymmetrical or far reduced elevation), and extremely severe decrease (no elevation). For the level of nasal sound released upon making an oral sound, the patient was instructed to pronounce a word with an oral sound and a sentence with an oral sound, and the rater subjectively assessed the level of nasal sound based on five stages—normal, weak nasality, moderate nasality, strong nasality, and very strong nasality. The words used in this assessment were “파파, 바바, 다다, 타타, 차차, 카카” (Fig. 2A) and the sentence was “학교 옆 바닷가에 파도가 거세게 쳐요” (Fig. 2B).
Vocal cord
The vocal cord function was assessed based on voice strength control and voice quality. For the voice strength control, the patient was guided to pronounce the numbers from one to 10, starting with the smallest possible voice and gradually increasing the voice to the loudest. The rater subjectively assessed the voice strength control based on five stages—normal, low-level abnormality, moderate-level abnormality, high-level abnormality, and very-high-level abnormality. For the voice quality, the spontaneous speech was induced from the patient, and the rater subjectively assessed the overall abnormality in sound intensity, pitch, tone, prosody, hoarseness, aspirated sound, tense sound, and strong nasality, based on five stages—normal, low-level abnormality, moderate-level abnormality, high-level abnormality, and very-high-level abnormality. The rater also left detailed comments on the voice quality and characteristics of the voice for the assessment, to allow an easy understanding by other investigators.
Respiration
The respiration function was broadly categorized into the strength and control of respiration, while the former was divided further into the induction of spontaneous speech, blowing an A4-sized paper, and maximal phonation time. The spontaneous speech was induced from the patient and the rater subjectively assessed the overall respiration pattern and intensity based on five stages—normal, low-level abnormality, moderate-level abnormality, high-level abnormality, and very-high-level abnormality. For blowing an A4-sized paper, the patient was instructed to blow a piece of a 15-cm wide A4-sized paper as further as possible (twice), and the longer duration was assessed based on the five stages—normal (≥12 seconds), slightly decreased (≥10 and <12 seconds), decreased (≥8 and <10 seconds), severely decreased (≥6 and <8 seconds), and extremely severe decrease (<6 seconds). For maximal phonation time, the patient was instructed to produce voice in a seated posture as long as possible twice, and the longer duration was recorded and assessed based on the five stages—normal (≥9 seconds), slightly decreased (≥7 and <9 seconds), decreased (≥5 and <7 seconds), severely decreased (≥3 and <5 seconds), and extremely severe decrease (<3 seconds). The control of respiration was assessed using repeated /hu/ (/후/) sound. The patient was instructed to make the /hu/ (/후/) sound for 5 seconds promptly, and the number of repetitions was assessed based on the five stages—normal (≥18 times), slightly decreased (≥15 and <17 times), decreased (≥12 and <14 times), severely decreased (≥9 and <11 times), and extremely severe decrease (≤8 times).
Articulation and phonation
The assessment of the articulation and phonation involved the use of sentence and word cards. The sentence cards contained sentences composed of 70 syllables, while the patient was guided to read each sentence as fast and accurately as possible. The rate and accuracy were assessed. For the rate, the time taken for reading the sentence was assessed based on the five stages—normal (<20 seconds), slightly decreased (≥20 and <22 seconds), decreased (≥22 and <24 seconds), severely decreased (≥24 and <26 seconds), and extremely severe decrease (≥26 seconds). For accuracy, the rater subjectively assessed the articulation based on five stages—normal (easy to understand all sentences), low-level abnormality (difficult to understand some sentences), moderate-level abnormality (difficult to understand most sentences), high-level abnormality (difficult to understand almost all sentences), and very-high-level abnormality (cannot un- derstand any sentences) (Fig. 2C). Lastly, the patient was shown eight word-cards and guided to pronounce each word, and the rater subjectively assessed the phonation based on the five stages—normal, low-level abnormality, moderate-level abnormality, high-level abnormality, and very-high-level abnormality) (Fig. 2D).
Statistical analysis
The scoring of the prototype test was based on the analysis of the result values obtained from 50 healthy individuals, from which the mean and standard deviation were calculated for each test item. The prototype test was performed for 50 patients with stroke. For examining the relative influence between the categories in the prototype test related to speech function and the U-TAP as a definitive diagnostic test, the odds ratio (OR) between the results of the prototype test and the results of the U-TAP was estimated. Through a regression analysis using the polychotomous linear logistic model, the OR and 95% confidence interval and the respective p-value were obtained, while the independent variables were the categories in the prototype test and dependent variables were the results of the U-TAP. The categories in the prototype test that exhibited OR≥1.00 as well as p≤0.05 were finally selected as the items composing the final test. Based on the relative level of OR of each of the finally selected items, the score of each test item was given a weighted value, and a diagnostic test for dysarthria with a total score of 100 was developed, where a high score on the final test indicated a high level of speech function. The sensitivity, specificity, positive predictive value, negative predictive value, and validity were obtained from the receiver operator characteristic (ROC) curve. The interand intra-rater reliability of the developed test was estimated using the intraclass correlation coefficient (ICC), and for the correlation among the total score on the final test, SMST, and U-TAP, the Spearman correlation coefficient was calculated. For all statistical analyses, the IBM SPSS Statistics 25 (IBM, Armonk, NY, USA) was used.
RESULTS
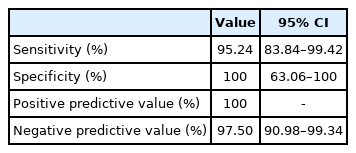
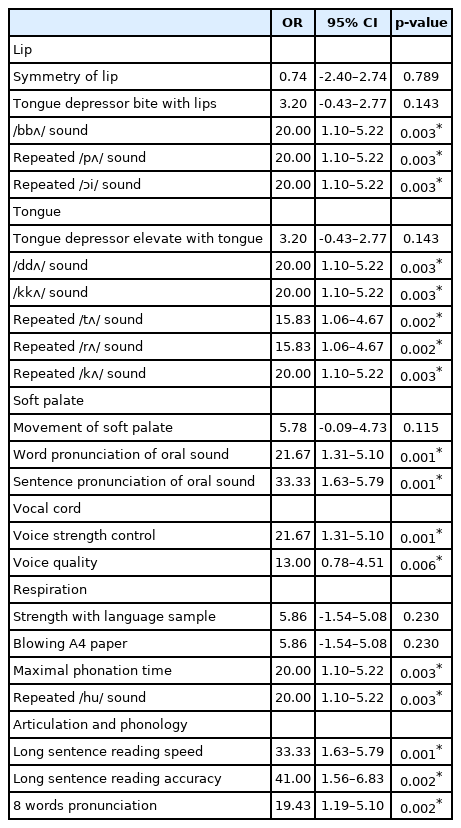
For each item of the prototype test and the definite diagnosis indicated by the U-TAP, the highest OR (41.00) was observed for the item of long sentence reading accuracy. Table 2 shows the OR values measured for each item of the prototype test for each speech organ. The 16 test items that showed OR≥1.00 and p≤0.05 were finally selected as the items for the final test. The scoring system was constructed so that an item with a low score indicates a reduced function of the corresponding organ, while a high total score indicates an overall moderate level of speech functions. Each item in the sub-categories were given a score based on the weighted value of OR (Appendix 1). At the cutoff point 76.50, the sensitivity, specificity, positive predictive value and negative predictive value of the KDT were as follows: 95.24%, 100.00%, 100.00% and 97.50% (Table 3). The area under the curve (AUC) obtained from the ROC curve was 0.99, indicating that the KDT was a test with high validity for dysarthria, while the confidence interval was 0.97–1.00. The Spearman correlation coefficient for the total score of the KDT and the SMST and for the total score of the KDT and the U-TAP were 0.49 (p<0.01) and 0.62 (p<0.01), respectively, to show a positive correlation with statistical significance. ICCs for inter- and intra-rater reliability were 0.75 and 0.60, respectively. No significant difference was found between patients with cerebral infarction and patients with cerebral hemorrhage. For the final test, the average time taken for the test was 16.61±1.25 minutes (Appendix 2).
Logistic regression analysis of associations between prototype test and dysarthria (prototype 23 items)
DISCUSSION
The prevalence of dysarthria is the highest among the neurogenic language disorders following stroke [1,2,9]. Although it is effective to detect and treat dysarthria early, there are few studies of stroke-related dysarthria. The purpose of this study is to develop a suitable assessment tool for stroke patients with dysarthria, with the focus on the early screening of the condition in stroke patients for better treatment.
The prototype test consisted of 23 items and these test items were systematically classified into six groups: lip, tongue, soft palate, vocal cords, respiration, articulation and phonological ability. Hence, the items including tongue depressor bite with lips, /bbʌ/ (/뻐/) sound, tongue depressor elevate with tongue, /ddʌ/ (/떠/) sound, /kkʌ/ (/꺼/) sound, movement of soft palate, voice strength control, respiration strength with language sample, blowing A4 paper, and maximal phonation time, were for the assessment of the strength of speech organs, while the items including repeated /pʌ/ (/퍼/) sound, repeated /Ɔi/ (/오이/) sound, /tʌ/ (/터/) repeat, /rʌ/ (/ 러/) repeat, /kʌ/ (/커/) repeat, word pronunciation of oral sound, sentence pronunciation of oral sound, voice strength control, voice quality, repeated /hu/ (/후/) sound, long sentence reading speed, long sentence reading accuracy, and eight words pronunciation, were for the assessment of the control of speech organs. Among these test items, the voice strength control can be used to assess both the strength and the control of speech functions.
The SMST, which is the most commonly used screening test of dysarthria in South Korea, differentiates the assessment of articulators into structure and function, from which a total score is obtained, while the result of the screening of phonation, voice, and articulation and of diadochokinesis are separately obtained; therefore, it is difficult for non-specialists to understand the patient’s speech function at a glance. Therefore, the present study reports the total score of the test results in numerical values as well as presenting the scores of each speech organ and function as a radar chart, so as to allow rapid review of the presence and severity of dysarthria in a patient and detect limitations at a glance. Furthermore, the constructed test items were easy and simple to allow anyone, including non-specialists, to perform the test with ease. In addition, as each speech organ was given a weighted value, the items with high contribution to dysarthria could be easily discerned, and as the scores were differentiated according to the relative weighted value, the validity and sensitivity could be improved.
The significance of this study lies in that a foundation for early treatment of dysarthria has been established through the development of a highly specific method of screening and diagnosis for dysarthria following stroke. In a clinical perspective, the findings in this study provide the data for an organ-specific approach, as each speech organ was assessed. The test also allows the severity of dysarthria to be predicted based on the total score, which could help in the treatment planning. What is of note is that the novel test in this study presents quantitative results by simplifying the extensive details of dysarthria test in 16 objective items, while allowing anyone to perform the test in a short period of time. Furthermore, the findings in this study indicated that the test of motor strength and control for the organs involved in the speech function led to the assessment of the comprehensive functions of the speech organs.
This study has several limitations and considerations. First, despite the efforts in this study to assess each test item as objectively as possible, the following items may contain the subjective opinion of the rater: word pronunciation in oral sound, sentence pronunciation in oral sound, voice strength control, quick pronunciation of a sentence, correct pronunciation of a sentence, and correct pronunciation of words. To improve objectivity, the test results were divided into five values according to severity. Second, the evaluation of dysarthria types is insufficient. Dysarthria is categorized into flaccid, spastic, ataxic, hypokinetic, hyperkinetic, unilateral upper motor neuron, and mixed types, based on the lesion and onset pattern [27]. In the early stage of planning the prototype test, the test items were constructed to reflect each specific type of dysarthria. However, this complicated the overall test structure and prolonged the testing time, so these items were not incorporated in the final test. To compensate for this limitation, the final test (KDT) included a margin in which the rater could record the detailed state and pattern of voice quality for reference in categorizing the type of dysarthria. Third, we did not consider signs of aspiration or dysphagia. Although inclusion of items to screen for aspiration signs was initially considered since previous studies reported that dysphagia could be related to dysarthria [28], more recent reports indicated no significant correlation between the two [29]. Fourth, 23 items of the prototype test were confirmed by two experts through content validity index scores, but the number of experts is fewer than recommended by the protocol [30]. Fifth, as this study focused on pure dysarthria following stroke, the potential outcome of the novel test in patients with dysarthria concurrently showing aphasia or apraxia could not be verified. Finally, the Spearman correlation coefficient between the KDT and the SMST is 0.49, and that between the KDT and the U-TAP is 0.62, which are relatively low. This is probably because, unlike the SMST or the U-TAP, the KDT was developed specifically for the assessment of dysarthria in stroke patients. Therefore, the KDT may be a more suitable test for these patients; this is anticipated to be confirmed through methods of complementation in follow-up studies.
CONCLUSION
In this study, a diagnostic test was developed to detect dysarthria and determine its severity. The test was performed for 50 healthy individuals and 50 stroke patients, and the results of each test item for the functions and organs related to speech were analyzed.
The stroke patients with dysarthria were shown to struggle to fulfill the instructions in the test for sentences rather than words or monosyllabic sounds. In addition, for each speech organ, the speech function of the subject was found to be better reflected in the soft palate and vocal cord functions than in the lip, tongue, and respiration functions.
The diagnostic tool developed in this study enables not only an objective and quantitative clinical assessment of dysarthria in stroke patients, but also an easy understanding of the effect of dysarthria on the function of each speech organ. The novel tool is also anticipated to assist in the early diagnosis of dysarthria as well as more focused treatment of the affected speech function. At present, the diagnosis of dysarthria in Korea relies on the U-TAP, but this test has not previously been performed for stroke patients, which highlights the significance of this study. Follow-up studies to identify the correlation between the novel test and the aforementioned conventional tests should be conducted so as to verify the utility of the novel test as a standard diagnostic tool for dysarthria in stroke patients.
Notes
No potential conflict of interest relevant to this article was reported.
Conceptualization: Lee KM. Methodology: Lee KM, Kim HJ. Data collection: Kim HJ. Formal analysis: Lee KM, Kim HJ. Funding acquisition: Kim HJ. Writing–original draft: Lee KM, Kim HJ. Writing–review and editing: Lee KM, Kim HJ. Approval of final manuscript: all authors.