Usefulness of the Simplified Cough Test in Evaluating Cough Reflex Sensitivity as a Screening Test for Silent Aspiration
Article information
Abstract
Objective
To assess cough reflex sensitivity using the simplified cough test (SCT) and to evaluate the usefulness of SCT to screen for silent aspiration.
Methods
The healthy control group was divided into two subgroups: the young (n=29, 33.44±9.99 years) and the elderly (n=30, 63.66±4.37 years). The dysphagic elderly group (n=101, 72.95±9.19 years) consisted of patients with dysphagia, who suffered from a disease involving central nervous system (ischemic stroke 47, intracerebral hemorrhage 27, traumatic brain injury 11, encephalitis 5, hypoxic brain damage 3, and Parkinson disease 8). The SCT was performed using the mist of a 1% citric acid from a portable nebulizer. The time from the start of the inhalation to the first cough was measured as the cough latency. All the dysphagic patients underwent the videofluoroscopic swallowing study.
Results
The cough latency was more significantly prolonged in the healthy elderly group than in the healthy young group (p<0.001), and in the dysphagic elderly group than in the healthy elderly group (p<0.001). The sensitivity and specificity of SCT were 73.8% and 72.5% for detecting aspiration in the dysphagic patients, and 87.1% and 66.7% for detecting silent aspiration in the aspirated patients.
Conclusion
Cough latency measured with the SCT reflects the impairment of cough reflex in healthy elderly and dysphasic subjects. The results of this study show that the SCT test can be a valuable method of screening aspiration with or without cough in dysphasic patients.
INTRODUCTION
Aspiration pneumonia is one of the serious complications of dysphagia [1]. Adequate swallowing reflex and the protective cough reflex in oropharyngeal swallowing are important in preventing aspiration pneumonia, and disruption or non-coordination of these reflexes are major risk factors for the development of the disease [2,3]. Many bedside screening methods of detecting or evaluating dysphagia have been suggested. Aside from evaluating the cause of dysphagia, it is important to assess the defensive action needed to eliminate foreign materials in the airway which are risk factors of silent aspiration. However, few tests have evaluated abnormal protective responses, such as impairment of the cough reflex and throat clearing [4].
Cough is one of the defensive reflex systems of the respiratory tract. It can be classified into the cough reflex, expiration reflex, and glottal closure reflex. Cough reflex is defined as a deep inspiration followed by closure of the glottis, forced expiratory effort, and then opening of the glottis with expiration. A cough promotes clearance of materials from the tracheobronchial tree and lungs [5]. It differs from expiration reflex, which consists of a forced expiratory effort with closure of the glottis, followed by opening of the glottis and the expulsive phase. While cough reflex is associated with both inspiratory and expiratory phases, expiration reflex is involved with a brief expiratory effort [6]. Throat clearing is a voluntary form of expiration reflex. Cough reflex also differs from glottal closure reflex, which brings out apnea caused by glottal adduction to laryngeal stimuli. Expiration reflex and glottal closure reflex from the larynx prevent aspiration of materials before proceeding into tracheobronchial tree and lung [5].
On the other hand, silent aspiration is caused by a lack of a strong reflex coughing or throat clearing when foreign materials are aspirated into the subglottic area [1]. In other words, if both the swallowing reflex and the protective cough reflex are impaired, there is a greater risk of developing aspiration pneumonia [7]. One study reported that silent aspiration was present in about 68% of the aspirating patients, according to a videofluoroscopic swallowing study (VFSS) [4]. Until now, VFSS and fiberoptic endoscopic evaluation of swallowing (FEES) have been considered to be the most accurate instrumental assessment tools for examining cough reflex as well as swallowing reflex. However, some patients cannot undergo sufficient medical examinations for the diagnosis of dysphagia, due to their general conditions and unavailability of such studies in some medical institutions. Supplementary screening tests for evaluating dysphagia in relation to aspiration have been investigated. These clinical screening methods have been reported with sensitivity of 70%-100% and specificity of 22%-66% compared to VFSS, in predicting aspiration [4]. Many current screening tests are based on the presence of cough for diagnostic criteria in detecting dysphagia. Therefore, it is possible that aspiration without cough might be missed. Several screening tests including clinical assessment, modified water tests, pulse oximetry test, and combination methods of these tests have been tried to detect silent aspiration. Some of the previous studies reported with 27%-100% in sensitivity and 39%-97% in specificity for detecting silent aspiration, on these supplementary tests in comparison to VFSS or FEES [8]. An evoked cough reflex test is also one of these efforts to supplement for clinical swallowing evaluation. The cough reflex can be evoked by mechanical or chemical stimulation. Chemical stimulation is challenged by an inhalation of tussive agents, such as citric acid, capsaicin, and tartaric acid. A number of previous researches concerning cough reflex evoked by tussive agents from conventional nebulizer measured the threshold concentration of the irritants [9]. Meanwhile, Sato et al. [10] suggested simplified cough test (SCT) and measured latency of cough reflex evoked by 1% citric acid from portable nebulizer. SCT is a simple method that is performed for only a few minutes while the patient is in a supine position. This test requires minimal patient cooperation, and it is less invasive and inexpensive. Therefore, SCT can be administered easily to patients confined to their bed or in the Intensive Care Unit. In spite of these advantages, there were only a few investigations conducted on SCT being used as screening test for dysphagia.
This study was conducted to assess cough reflex sensitivity in dysphagic patients, using the SCT with 1% citric acid, and to compare them with that of healthy subjects. Also, the usefulness of SCT for detecting silent aspiration in dysphagic patients was evaluated. While Sato et al. [10] used the results of the FEES as the standard in identifying silent aspiration, we use the VFSS as the standard for this study.
MATERIALS AND METHODS
Subjects
This study was conducted at the rehabilitation unit of Chung-Ang University Hospital in Seoul, South Korea. The healthy control group that had no symptoms of dysphagia was divided into two subgroups [11,12]: the young adult group (group 1: n=29, female, 33.44±9.99 years) and the elderly group (group 2: n=30, female, 63.66±4.37 years). They were recruited via public postings at the campus of Chung-Ang University College of Medicine. Many factors are known to influence the sensitivity of the cough reflex, which include age, gender, smoking, and pulmonary disease [5,13,14]. In healthy control group study, we focused on normative values for cough sensitivity, so the exclusion criteria were quite comprehensive. The exclusion criteria included the history of smoking, asthma, head and neck cancer, head and neck surgery, airway diseases, suggestive symptoms within 4 weeks, respiratory tract infection, and seasonal allergies. In addition, the subjects did not take any medications regularly. During the recruitment, there were a number of male subjects who met the exclusion criteria of being smoker, and those were excluded. For this reason, we focused on the female population for this study [11,13,14].
The dysphagia group (group 3: n=101, female, 72.95±9.19 years) had swallowing difficulties caused by several diseases of the central nervous system (ischemic stroke 47, intracerebral hemorrhage 27, traumatic brain injury 11, encephalitis 5, hypoxic brain damage 3, and Parkinson disease 8). To increase comparability between the control and dysphagia group, this study was performed on female dysphagic patients. The patients recruited were those referred and admitted to rehabilitation unit or those who requested for a consultation on their dysphagic symptom. The written informed consent, for assessment of their swallowing through the citric acid inhalation test, was obtained from all of the subjects. This study was approved by the Chung-Ang University Hospital Institutional Review Board.
Methods
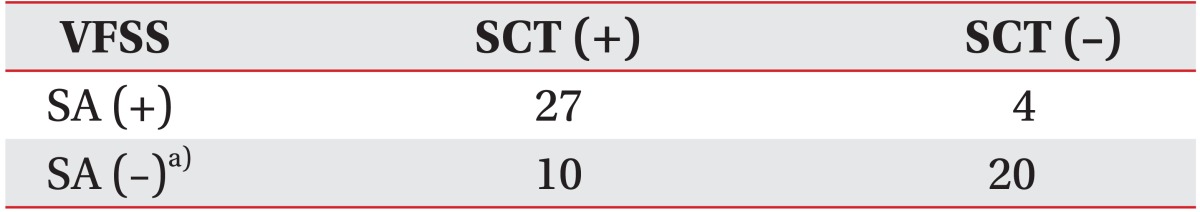
SCT [10] was performed in all subjects using a mist of a 1% w/v citric acid-physiological saline solution from a commercially purchased portable nebulizer and mouthpiece (NE-U22; Omron, Kyoto, Japan). The citric acid mist was administered until the first cough. The time from the start of the inhalation to the first cough was measured as cough latency in seconds. If the subject did not cough for one minute, the test was ended and the subject was confirmed as a non-cough subject (Fig. 1). All the subjects in group 3 underwent VFSS within 2 days after SCT. At the medical institute of the authors, VFSS is performed in a fluoroscopic laboratory by an experienced rehabilitation physician assisted by a radiologic technician. The subjects were asked to sit on a chair and turn 90° to be in the lateral projection position. Then, they were given 2 mL of water, followed by 2 and 5 mL of a thin-fluid mixture with barium, using a syringe. Next, they were given 2 to 3 mL of boluses consisting of nectar, honey, rice porridge, and cooked rice, and dual-consistency boluses consisting of a mixture of cooked rice and barium diluted fluid, using a spoon. Finally, 30 mL of thin fluid was administered using a cup. All the trials were performed twice, and the VFSS results were reviewed. Supraglottic penetration or laryngeal penetration was defined as a presence of contrast material in the laryngeal vestibule, which is above the true vocal cord. Subglottic aspiration or tracheobronchial aspiration was defined as a presence of contrast material below the true vocal cord [1]. In this study, only subglottic aspiration was classified as an aspiration. Silent aspiration was defined as aspiration in the absence of a reflexive cough response [1].
Statistical analysis was performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was used to compare the cough latency values among the groups. Post-hoc tests were conducted using Duncan method. The value was obtained by adding the specificity and sensitivity to a peak with coordinates in the farthest point from the baseline vertically, when the receiver operating characteristic (ROC) curve reached the maximum. This was set as the cutoff value of the cough latency in SCT for the dysphagic patients. The p-value of less than 0.05 was considered to be statistically significant.
RESULTS
Cough latency of SCT in the healthy and dysphagic groups
All of 160 subjects consisting of 59 healthy people and 101 dysphagic patients completed the experiments without any side effect. The results of the SCT are shown in Table 1 and Fig. 2. The cough latency was significantly longer in group 2 (14.91±16.62 seconds) than in group 1 (3.96±3.92 seconds) (p<0.001), and significantly longer in group 3 (29.61±22.23 seconds) than in groups 1 and 2 (p<0.001) (Table 1, Fig. 2).

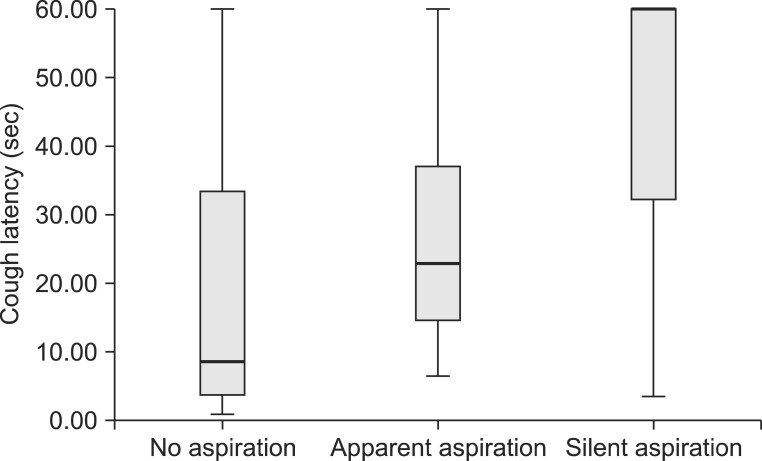
Cough latency of all the experiment groups. Data are presented as box-plots, where the bottom and top of the box are the first and third quartiles, and the horizontal line inside box is median value. The ends of the whiskers the minimum and the maximum value within group. Small filled circle (•) and filled upward triangle (▴) represent outliers which are not included between the whiskers.
Comparison of the SCT and VFSS results in the dysphagic group
According to the VFSS results, the subjects in group 3 were categorized into three subgroups: the non-aspirator, apparent aspirator, and silent aspirator. The SCT results in group 3 are shown in Table 2. According to the VFSS results, 40 patients did not show aspiration and 30 patients showed both aspiration and cough. The remaining 31 patients were categorized into silent aspiration group, because subglottic aspiration was observed during VFSS with the absence of cough. The cough latency was significantly longer in the apparent aspiration group (27.24±18.22 seconds) than in the no aspiration group (17.99±19.57 seconds) (p<0.001). In addition, the cough latency was significantly longer in the silent aspiration group (46.90±18.28 seconds) than in the aforementioned two groups (p<0.001) (Table 2, Fig. 3).
ROC curve of the SCT for screening subglottic silent aspiration
ROC curve analysis was performed to reveal the cough latency with reasonable sensitivity and specificity. The cutoff value of the cough latency for the aspiration including both apparent and silent aspiration in the dysphagic patients was 20.42 seconds (confidence interval [CI]=68.2%-87.3% and area under the ROC curve [AUC]=0.778). Using this SCT cutoff, cough within 20.42 seconds was considered to be negative or normal, while cough over the time or non-cough was regarded as positive. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for detecting aspiration in all dysphagic patients were 73.8%, 72.5%, 80.4%, and 64.4%, respectively (Table 3, Fig. 4). The cutoff value of the cough latency for silent aspiration in aspirated patients was 28.12 seconds (CI=66.2%-90.3% and AUC=0.783). Using this SCT cutoff, cough within 28.12 seconds was considered to be negative or normal, while cough over the time or non-cough was regarded as positive. The sensitivity, specificity, PPV, and NPV for detecting the silent aspiration in the aspirated patients were 87.1%, 66.7%, 73.0%, and 83.3%, respectively (Table 4, Fig. 5). The cutoff value of the cough latency for silent aspiration in all dysphagic patients was 28.12 seconds (CI=72.9%-91.1% and AUC=0.820). Using this SCT cutoff, cough within 28.12 seconds was considered to be negative or normal, while cough over the time or non-cough was regarded as positive. The sensitivity, specificity, PPV, and NPV for detecting silent aspiration in all dysphagic patients were 87.1%, 70.0%, 56.3%, and 92.5%, respectively (Table 5, Fig. 6).

Results of the SCT for detecting aspiration in all the dysphagic patients using 20.42 seconds as the cutoff
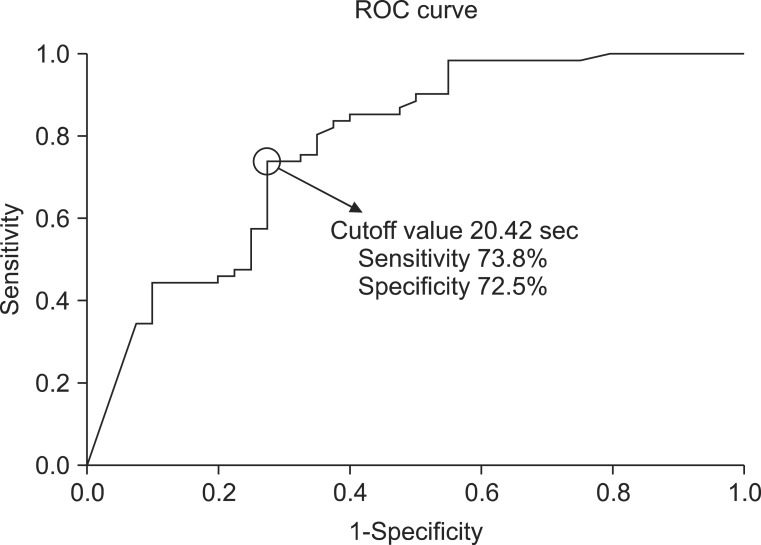
Receiver operating characteristic (ROC) curve analysis of the simplified cough test with respect to its ability to detect aspiration in all the dysphagic patients.
Results of the SCT for detecting silent aspiration in the aspirated patients using the 28.12 seconds as the cutoff
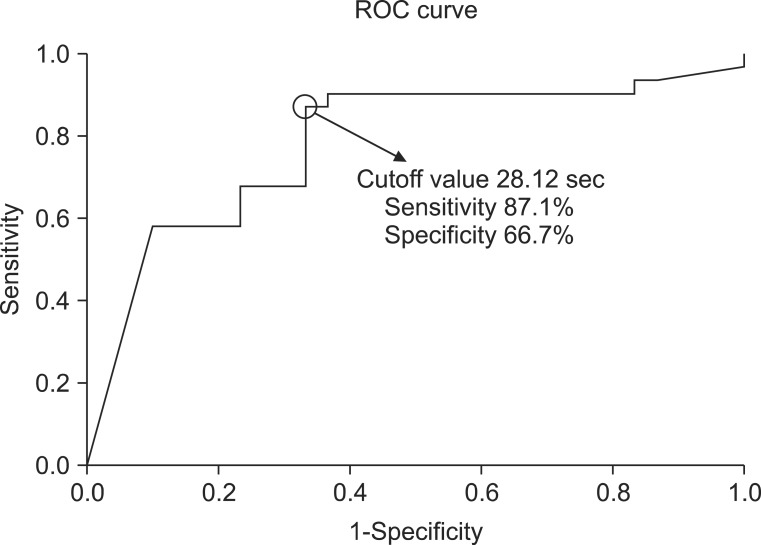
Receiver operating characteristic (ROC) curve analysis of the simplified cough test with respect to its ability to detect silent aspiration in the aspirated patients.
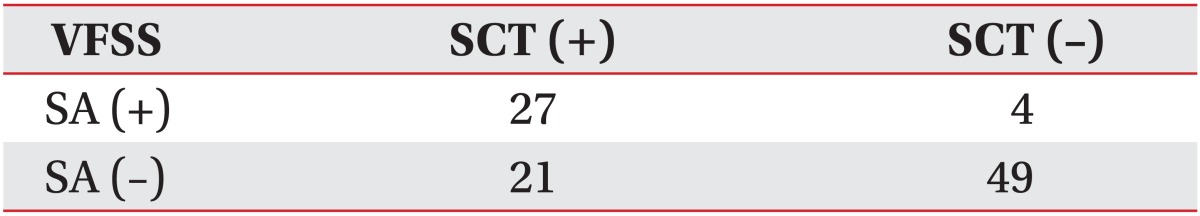
Results of the SCT for detecting silent aspiration in all the dysphagic patients using the 28.12 seconds as the cutoff
DISCUSSION
A cough reflex system basically involves airway afferent nerves and receptors, and the brainstem including cough center, cerebral cortex, and efferent pathways. The airways are innervated by vagal afferent nerves and have receptors or nerve terminals, such as bronchopulmonary rapidly adapting receptors (RARs) and bronchopulmonary C-fibers that initiate coughing. RARs are sensitive to mechanical stimulation and deformation of airway epithelium, such as airway smooth muscle contraction and airway wall edema, due to particulate matter, mucus, or constrictor agents. These air mechanoreceptors are predominantly present in the larynx, trachea, and carina. RARs also respond to acids including citric acid, low-chloride solutions, and distilled water. In contrast, bronchopulmonary C-fiber nociceptors are sensitive to chemicals, such as bradykinin, capsaicin, and other classical nociceptor stimulants. Nociceptors are generally quiet in normal airways but are recruited during inflammation or irritation. Mechanoreceptors are insensitive to direct chemical stimuli, such as bradykinin and capsaicin. However, these chemicals can activate mechanoreceptors by inducing secondary mechanical change through airway smooth muscle contraction, mucus, or edema [15]. Next, afferent vagal fibers for cough enter the cough center of the medulla in the brainstem and relay a few fibers by connecting with the second-order neuron for interaction with cerebral cortex. In addition, these afferents are hypothesized to be projected to cerebral cortex, including to urge-to-cough neural network [15,16].
Cough is a complex and highly coordinated reflex. Therefore, any problem in cough reflex pathway can impair cough generation and increase the risk of silent aspiration. In this study, we focused on evaluating the functional status of peripheral respiratory tract sensation, as it initiates the cough reflex pathway. In this respect, the objective motor behavior of cough as output of afferent transduction was considered to be important. The results of this study showed that the cough reflex latency in SCT using citric acid was delayed significantly in healthy elderly people compared to healthy young people. This result is consistent with that of the previous studies [12]. The underlying cause of the reduction of cough reflex sensitivity in elderly subjects is unclear, but it is likely to be multi-factorial. In healthy subjects without a central nervous system (CNS) problem, such as stroke, Parkinson disease, and other CNS disorders, the peripheral afferent and efferent system would be considered as the cause of impaired cough reflex [5,12,15]. Cough reflex sensitivity was also reduced significantly in dysphagic elderly patients compared to healthy elderly people. Majority of the subjects in the dysphagic groups were CNS-injured patients. The reduced cough reflex sensitivity and sensory impairment of the airway may significantly affect elderly people and dysphagic patients, and these may bring about a greater risk for silent aspiration or aspiration pneumonia in clinical situations. As already mentioned, the underlying causes of the down-regulation of cough reflex are likely to be multi-factorial, in addition to sensory impairment of oropharynx area. It is known that cough reflex exhibits plasticity at sensory receptor, afferent ganglion, and central nervous levels, and the regulation of each component showed a different process in various physiologically or pathologically induced situations. In addition to sensory defect, induction of cough is also associated with control of respiratory motor pathway [5,17].
The SCT showed moderate sensitivity and specificity in the detection of aspiration. SCT was originally designed to detect silent aspiration, or aspiration without cough. According to the results of this study, SCT effectively detects aspiration including apparent aspiration and silent aspiration. This is consistent with the previous view of association between aspiration and impaired cough reflex [3,18]. Within the dysphagic patients (group 3), a few non-aspirators did not cough, which is corresponding to a false positive with SCT. This proportion is one cause of the reduced sensitivity and specificity of SCT as a screening tool for detecting aspiration. Many of these people did not have overt aspiration, but laryngeal penetration was observed in VFSS. Laryngeal penetration can definitely be observed in normal individuals. However, dysphagic patients who showed laryngeal penetration without cough are considered to be potentially at greater risk of silent aspiration or aspiration pneumonia. Therefore, the dysphagic patients who showed positive SCT results without definite aspiration should be closely observed through follow-ups.
The SCT showed high sensitivity and moderate specificity for the detection of silent aspiration, which matched well to our expectations of its effectiveness as a screening tool. In the previous research using the same SCT protocol, Sato et al. [10] suggested the coughing within 30 seconds as a cutoff value to detect silent aspiration from the aspirator (sensitivity 92%, specificity 94%) and 60 seconds for all dysphagic patients (sensitivity 81%, specificity 65%). In this study, we obtained 28.12 seconds for predicting the silent aspiration from aspirator cohort (sensitivity 87.1%, specificity 66.7%). This was consistent with the previous study [10]. However, we found that the 28.12 seconds rather than 60 seconds was sufficient to predict silent aspiration for dysphagic patients cohort (sensitivity 87.1%, specificity 70.0%) as well as aspirator cohort. If the cutoff value is increased, it is possible that more subjects may be shown to cough. As a result, the specificity will be higher but the sensitivity will be lower. Generally, for disease with high-risk or high prevalence, tests with a higher sensitivity are preferred than those with a higher specificity. They are better at ruling out disease that has fewer false negative [19]. As mentioned above, silent aspiration does not happen infrequently and has a close relation to developing aspiration pneumonia. Therefore, 28.12 seconds is more appropriate to detect silent aspiration in dysphagic patients.
The sensitivity and specificity values for aspirator were lower than those in previous study by Sato et al. [10]. We speculate that there were several possible reasons for these differences. First, there were differences in patient population. Our study was performed in female population to ensure comparability and remove the confounding effect of smoking. In contrast, there was higher proportion of men than women in the previous study [10]. Most of the patients with aspiration were men, and there were a few women among the patients with silent aspiration. It was reported that women show lower threshold and higher sensitivity of cough reflex than men [14]. Therefore, it is likely that more people with prolonged cough latency or lower sensitivity of cough reflex to tussive agent were included in their previous study [10], compared to the population in our study. In addition, we performed SCT in dysphagic patients who were admitted by CNS impairments. Most of them were in acute to subacute stage. Otherwise, there were more heterogeneous populations of dysphagic patients in the study by Sato et al. [10], including disuse syndrome, respiratory disease, cancer, cervical spine injury, as well as neurological disorder. Furthermore, there was no detail about duration or state of disease from which the subjects suffered. Therefore, it is difficult to simply compare the results from the study by Sato et al. [10] with our findings. Second, the difference in standard instrumental assessment of swallowing needs to be considered. We used VFSS as a gold standard method to judge silent aspiration, while FEES was conducted for identifying silent aspiration in their study. VFSS and FEES are both established instrumental tools for identifying normal and abnormal swallowing physiology and identifying presence and absence of aspiration [20]. According to some studies, FEES can identify trace amounts of penetration and aspiration that are not visible on VFSS [21]. It is questionable whether these abnormal findings that are only detected by FEES have a clinical relevance, because silent aspirations are detected more in the subjects who show impaired cough reflex. The sensitivity of SCT would have been increased from the point of validity study. The third possible cause is related to judgment on weak cough response. In this study, we regarded even the weak cough as negative or normal at SCT. There was no documentation on judgments about weak cough or ambiguous cough in the previous studies [10]. Generally, a test with high sensitivity and low specificity is more preferable as a screening test, to the one with low sensitivity and high specificity. If weak cough was classified as positive or abnormal at SCT, the sensitivity of detecting silent aspiration would be improved. Although it is not clear whether a weak cough reflex represents a reduced sensory response to irritants or a weakened motor response, there is no doubt that weak cough responding to irritants increases risk of aspiration.
This study has several limitations. First, SCT was performed only in female populations. It is known that many factors including age, gender, smoking, and asthma influence the sensitivity of cough reflex [5,13,14]. Before the SCT is applied into clinical dysphagia assessment, normative data should be established in the non-smoking young and elderly population. During the recruitment, there were a number of male subjects who were smokers. We aimed to establish a normative data in control group and exclude influences from smoking factors, so we focused on female population in this study. However, to use SCT clinically in all population, further studies should gather more subjects including males and investigate influences of smoking. Second, we used 1% citric acid as a tussive agent based on the results from Sato et al. [10]. We did not test at lower or higher concentration, and there is a possibility that some subjects would have triggered to cough at lower or higher than 1% citric acid. Further research is warranted to gather normative data using SCT method, on the threshold concentration that triggers cough in healthy subjects. The studies in healthy population will be followed by dysphagic patients. Third, we did not measure the cough strength, and we decided the presence of cough based on the clinician. Assessment of the strength of cough reflex may be valuable in assessing the ability to clear aspirated materials. There has been literature published on the objective measures, such as airflow and subjective judgment of cough strength. The judgments on subjective cough strength can be provided through training by clinician or speech language therapist [22]. A reliable cough reflex test with a subjective strength rating may be more beneficial to clinical swallowing evaluation, and it can be used as bedside screening test. Therefore, future research on the SCT including subjective cough strength rating is necessary.
In conclusion, we compared the cough sensitivity in healthy and dysphagic patients with SCT, using citric acid inhalation. The result of SCT showed significant delay in the elderly population. It also showed high sensitivity and moderate specificity for detecting silent aspiration in the patients with dysphagia, which could reflect impairment of cough reflex in elderly people and dysphagic patient. From these findings, SCT can be a useful screening method to detect or predict the aspiration with or without cough in dysphagic patients.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (No. A100054).
Notes
No potential conflict of interest relevant to this article was reported.