Motor Function in the Late Phase After Stroke: Stroke Survivors’ Perspective
Article information
Abstract
Objective
To examine the association between observer-assessed functional status and perceived recovery in the late phase after stroke. The study also aimed to determine whether observer-assessed functional improvements as a result of horse-riding therapy (H-RT) are related to enhanced perception of stroke recovery.
Methods
This is a descriptive correlational study using data derived from a three-armed randomized controlled trial in which 123 individuals were enrolled, among whom 43 received H-RT for 12 weeks. The measures included the Modified Motor Assessment Scale, Berg Balance Scale, Timed Up and Go, timed 10-m walk, and perceived recovery from stroke indicated by item #9 in the Stroke Impact Scale (version 2.0). Spearman rank order correlation (rs) was used in the analyses.
Results
There were moderate to strong positive or negative correlations between all four observer-assessed motor variables and participants’ ratings of perceived late-phase stroke recovery at trial entrance, ranging from rs=-0.49 to rs=0.54 (p<0.001). The results of the correlational analyses of variable changes showed that, after the end of the H-RT intervention, both self-selected and fast gait speed improvement were significantly correlated with increments in self-rated stroke recovery (rs=-0.41, p=0.01 and rs=-0.38, p=0.02, respectively).
Conclusion
This study provided data supporting the association between individual ratings of self-perceived recovery after stroke and observer-assessed individual motor function. The results further demonstrate that enhancement in perceived stroke recovery after completing the intervention was associated with objectively measured gains in both self-selected and fast gait speed.
INTRODUCTION
Stroke is a heterogeneous condition with complex symptomatology, and despite recent advances in treatment and rehabilitation, the condition often leads to persistent disability [1]. Many stroke survivors have motor impairment [2]. Balance deficits, spasticity, and dyscoordinated gait due to hemiparesis dispose stroke survivors to sedentary behaviors, which hamper postural control ability and increase the risk for falls [3-5]. Impaired mobility may also lead to significant activity limitations and participation restriction [1,6], thereby increasing social isolation and loneliness [7-9]. Even though rehabilitation programs for patients with stroke include gait training, many individuals still have motor impairment after completion of standard rehabilitation. Improved walking ability is a prioritized goal among stroke survivors, and recovery of impaired motor functionsis a major aspect of stroke rehabilitation [2].
We have previously found that horse-riding therapy (H-RT) and rhythm- and music-based therapy (R-MT) helps promote subjective and objective functional recovery in the late phase after stroke [10,11], including gains in motor function. Additional in-depth analyses of data derived from the randomized controlled trial (RCT) supported the observed efficacy of H-RT in producing immediate and sustained gains in gait and functional mobility in the late phase after stroke, whereas the effectiveness of R-MT in this respect was less convincing [12].
Evaluation of individual recovery following stroke is critical for both treatment and research purposes [13]. Functional assessments reveal that certain aspects of individual dysfunction are related to the degree of recovery, such as neurological deficit (e.g., hemiparesis or aphasia), performance of specific tasks (e.g., feeding oneself or walking), function in normal roles and activities (e.g., employment or hobbies), and quality of life [14]. Patient-reported outcome measures (PROMs) are useful in addition to observer-assessed measures in assessing health status of individuals with stroke [15]. In some cases, PROMs may even be better than observer-assessed measures in identifying changes in health status of relevance for the patient [16]. However, whether the degree of observer-assessed motor recovery in the late phase after stroke is associated with the degree of perceived stroke recovery is unclear.
Previous studies have demonstrated that gait speed is a reliable, valid, and sensitive measure of recovery after stroke that reflects impaired mobility and functional community walking ability [17-19]. Gait speed is also a common indicator used to predict future health status and healthcare utilization among older adults [20,21]. It has been demonstrated that improvements in walking speed are correlated with improved participation and quality of life [18,22]. Moreover, improvement in walking speed may reflect a genuine improvement in mobility, even if other measures fail to detect it [23]. This study aimed to assess the extent to which subjective ratings of perceived recovery after stroke are associated with observer-assessed functional status. It also aimed to determine whether observer-assessed functional improvements resulting from H-RT could be linked to enhanced perception of stroke recovery.
MATERIALS AND METHODS
Study design and participants
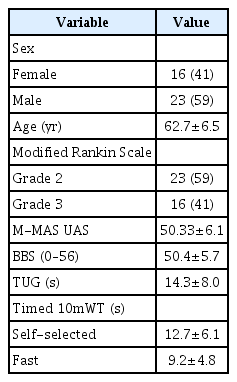
This is a descriptive correlational study using data derived from a three-armed RCT that assessed whether R-MT and H-RT could lead to increased recovery in the late phase after stroke [10,11]. The RCT enrolled 123 participants, the eligibility criteria were limited to individuals who had stroke between 10 months and 5 years prior to inclusion in the trial who had disability/dependence equivalent to grades 2 and 3 on the modified Rankin Scale (mRS).
The observer-assessed measurements of motor function and gait were conducted by a trained physiotherapist, and a subset of the data collected at baseline was used to study the association between functional status and perceived recovery after stroke (n=123). Data describing the change in observer-assessed functional measures at intervention completion and 6-month follow-up was used to determine whether an increase in perceived stroke recovery was associated with gains in functional measures in the H-RT group (n=43). All participants provided written informed consent. Consenting individuals attended an appointment with a specialist in rehabilitation medicine for a neurological screening assessment and interview prior to inclusion. Ethical approval was granted by the Regional Ethical Review Board in Gothenburg (No. 698-09), and the study was conducted in accordance with relevant ethical guidelines.
Measures
Stroke severity and disability
To objectively quantify stroke-related neurologic deficits, the National Institutes of Health Stroke Scale (NIHSS) was used. The NIHSS was originally designed as a research tool to measure baseline data inpatients of acute stroke clinical trials but is now also widely used as a clinical routine assessment tool. The NIHSS has been shown to be reliable and predictive of both short- and long-term outcome following stroke [24,25].
Perceived stroke recovery
Prior to randomization in the RCT, the global perception of recovery from stroke was evaluated using item #9 in the Stroke Impact Scale (SIS, version 2.0). The item was presented in the form of a visual analogue scale, and participants were asked to rate their recovery on a scale of 0 to 100, with 100 representing full recovery. The SIS can be used in both clinical and research settings and is shown to be valid, reliable, and sensitive to change [26].
Observer-assessed balance function
Balance ability was evaluated using Berg Balance Scale. The test contains 14 elements, adding up to a total score (maximum 56). A score <45 may indicate an increased risk of falling and therefore the need of assistance. The test has high validity, reliability, and inter-and intra-rater reliability [27].
Observer-assessed mobility and gait
Basic functionalmobility was assessed using the Timed Up and Go (TUG). The time taken to complete the TUG was measured in seconds using a stopwatch. The TUG is a well-documented test of functional balance with high validity and reliability [28].
Gait speed was assessed using the timed 10-m walk test [29]. The test was performed in a straight corridor from a standing start with the participant positioned behind a starting line marked with tape on the floor. The timer was started once the subject initiated walking and their first foot passed the starting line. The timer was stopped when the subject’s first foot passed the end of the 10-m pathway marked with tape. Walking time was measured in seconds for both self-perceived comfortable pace and fast pace. Walking aids were allowed, but the same aids had to be used on all test occasions. No physical assistance was permitted. The test is considered to have high interrater and test-retest reliability and low variability [30,31].
Observer-assessed functional mobility performance
Daily motor function was assessed using the Modified Motor Assessment Scale according to Uppsala University Hospital (M-MAS UAS) [32]. The scale was developed based on Carr and Shepherd’s theories of motor relearning after stroke [33]. The scale was based on performance and assessed performance of functional tasks rather than isolated patterns of movement. It has been modified since 1991 and validated in different studies. In this study, the M-MAS UAS version 1999 was used. The MMAS UAS was used to assess eight motor components in individuals with stroke: supine to side lying, supine to sitting over side of bed, sitting, sitting to standing, walking, upper arm function, hand movements, and fine motor activities. The latter three components were assessed bilaterally. Each item was scored from 1 to 5; the maximum score of 55 indicates optimal motor function. Motor control was measured in eight levels. To provide a global perspective of patients’ functional mobility capacity, the total M-MAS score was used.
Statistical analysis
Descriptive statistics were used for demographic characteristics of the sample. Categorical variables were presented as number and percentage. Continuous variables were described using mean and standard deviation (SD). To explore the association between observer-assessed functional statusand participants’ perception of stroke recovery at baseline in the RCT, Spearman rank-order correlation was used. The strength of the relationships was classified as low (r<0.30), moderate (0.30≤r≤0.50), and high (r>0.50) [34]. Spearman rank-order correlation was also used in investigating whether observer-assessed functional improvements following H-RT are related to increments in self-rated stroke recovery after treatment. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 24.0 (IBM Corp., Armonk, NY, USA). All statistical tests were two-tailed, and a p-value less than 0.05 was considered statistically significant.
Intervention
The H-RT was performed at a riding center purpose-built for the disabled where trained therapy horses were used. Participants attended twice weekly sessions of H-RT during a 12-week intervention period. The H-RT was designed to stimulate motor, sensory, cognitive, emotional, and social functions. A description of the H-RT content and expected therapeutic benefits has been previously presented in detail [10,11].
RESULTS
The study cohort of 123 individuals (44% women and 56% men) had a mean age of 63 years (SD=6.5). The mean elapsed time since stroke onset for the study group was 1.156 days (SD=484). Demographics and clinical characteristics are presented in Table 1. The NIHSS scores indicated mild to moderate stroke severity, with a mean score of 2.77 (SD=2.81). Table 2 presents the Spearman correlation coefficients describing the association between the observer-assessed functional measures and perceived stroke recovery at baseline. There were moderate to strong correlations between all motor variables and perception of stroke recovery, among which the performance of daily motor tasks (M-MAS) was most strongly related to perceived recovery (rs=0.54; p<0.001).

Spearman correlation coefficients describing the association between observer-assessed motor function and perceived stroke recovery at baseline of the intervention study
Demographics and clinical characteristics of the participants who were enrolled in the H-RT group are presented in Table 3. The NIHSS scores of the H-RT group indicated mild to moderate stroke severity, with a mean score of 2.62 (SD=3.18). The correlational analyses of thevariable changes showed that, at post-intervention, both self-selected and fast gait speed improvement was significantly correlated with increments in self-rated stroke recovery (Table 4). In Fig. 1, scatter plots for the association between gains in gait speed and increments in perceived stroke recovery at the end of treatment are shown. The correlations between changes in these variables were no longer significant at the 6-month follow-up.
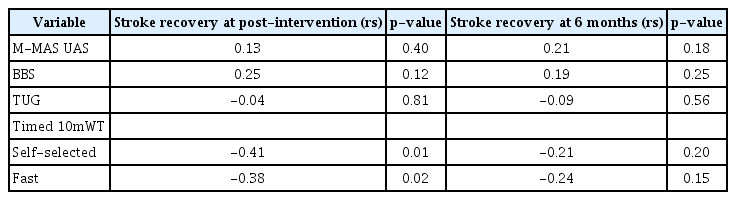
Spearman correlation coefficients describing the association between therapy-induced gains in motor function and perceived increments in stroke recovery after receiving horse-riding therapy
DISCUSSION
The findings of the present study demonstrated that the degree of observer-assessed motor function among latephase stroke survivors enrolled in a clinical study could be related to subjective ratings of perceived recovery since the stroke onset. It is of interest that a subjective global measure of recovery is associated with the degree of persistent motor deficitsin the late phase after stroke. Daily motor function measured using M-MAS UAS was linked to the perception of recovery, as was balance and gait ability. Hence, the ability to perform functional tasks and isolated patterns of movement or postural control functions, such as balance and gait, seem to be related to perceived disability among stroke survivors.
Furthermore, data in the present study also provided the opportunity to assess the longitudinal relationship between perceived stroke recovery and observerassessed functional improvements after an intervention. These complementary analyses showed that changes in the individual’s perception of recovery after completing the 12-week H-RT intervention was associated with objectively measured gains in both self-selected and fast gait speed. Participants with greater gait-speed improvement reported greater perceived recovery compared to participants with less gait speed gains after the H-RT. This finding highlights the importance of using interventions designed to improve walking ability, not only in the acute and subacute phases after stroke but also in later stages of the disease. Improved gait ability has been described as one of the most important goals in individuals with stroke undergoing rehabilitation [35] and those living with stroke in the community [35]. The ability to voluntarily increase gait speed from slow to fast speed helps stroke survivors to adapt better in activities of community life, such as crossing a street and facing unpredictable pedestrian and street traffic [36].
The potential limitations of this study should be considered when interpreting the results. One of these limitations are the eligibility criteria in the RCT, restricting our study population to individuals with moderate disability after stroke (mRS grades 2 and 3). This limits extrapolation of our findings to a population of stroke survivors with either very mild (mRS 1) or more severe disability (mRS 4). In contrast, the study enrolled a mixed population of late-phase stroke survivors who had completed conventional rehabilitation programs and were living at home with chronic stroke-related disability.
Therefore, this study demonstrates an association between individual ratings of self-perceived stroke recovery and observer-assessed individual motor function in the late phase after stroke onset.The results further demonstrate that enhancement in perceived recovery among late-phase stroke survivors after completing a 12-week H-RT intervention was associated with objectively measured gains in both self-selected and fast gait speed.
Acknowledgements
This work was supported by grants from Sten A Olsson Foundation for Research and Culture, the Swedish Brain Foundation, the Swedish Arts Council, the Swedish state under the agreement between the Swedish government and county councils, the ALF agreement (No. ALFGBG-716591, ALFGBG-146051), AFA Insurance, the Swedish Stroke Association, Rune and Ulla Amlöv’s Foundation for Neurological and Rheumatological Research, Edith Jacobson Foundation, Per-Olof Ahl Foundation for Neurological Research, Sigurd and Elsa Goljes Memorial Foundation, Wilhelm and Martina Lundgren Scientific Foundation, Doktor Felix Neubergh’s Foundation, the Swedish Society of Medicine, and the Foundation for Rehabilitation and Medical Science.
Notes
No potential conflict of interest relevant to this article was reported.
Conceptualization: Bunketorp-Käll L, Samuelsson H. Formal analysis: Bunketorp-Käll L. Writing - original draft: Bunketorp-Käll L. Writing - review and editing: Pekna M, Pekny M, Samuelsson H, Blomstrand C, Nilsson M. Approval of final manuscript: all authors.