Clinical Importance of Peak Cough Flow in Dysphagia Evaluation of Patients Diagnosed With Ischemic Stroke
Article information
Abstract
Objective
To investigate the relationship between peak cough flow (PCF), pulmonary function tests (PFT), and severity of dysphagia in patients with ischemic stroke.
Methods
This study included patients diagnosed with ischemic stroke, who underwent videofluoroscopic swallowing study (VFSS), PCF and PFT from March 2016 to February 2017. The dysphagia severity was assessed using the videofluoroscopic dysphagia scale (VDS). Correlation analysis of VDS, PFT and PCF was performed. Patients were divided into three groups based on VDS score. One-way ANOVA of VDS was performed to analyze PCF, forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and age among the different groups.
Results
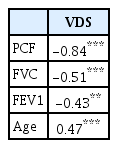
The correlation coefficients of VDS and PCF, VDS and FVC, and VDS and FEV1 were -0.836, -0.508, and -0.430, respectively, all of which were statistically significant at the level of p<0.001. The one-way ANOVA indicated statistically significant differences in PCF, FVC, FEV1, and age among the VDS groups. Statistically significant differences in VDS and age were observed between aspiration pneumoia and non-aspiration pneumonia groups.
Conclusion
Coughing is a useful factor in evaluating the risk of aspiration in dysphagia patients. Evaluation of respiratory and coughing function should be conducted during the swallowing assessment of patients with ischemic stroke.
INTRODUCTION
Dysphagia occurs in 30% to 50% of all stroke patients. The normal swallowing process is a complex sensory motor behavior involving several muscles controlled by the central nervous system and the peripheral nervous system working in sequence [1,2]. Acute stroke occurring in the cerebral, cerebellar or brain stem triggers dysphagia by inducing dysfunction in normal swallowing physiology.
Because swallowing and respiratory systems share the aero-digestive tract as an anatomically common area, dysphagia in acute stroke patients increases the risk of aspiration pneumonia and extends hospitalization periods. Aspiration pneumonia is known to develop in 20% to 30% of stroke patients [3,4].
Several studies have shown that cough and respiratory functions are physiologically associated with swallowing ability. Voluntary coughing in particular is a factor for the direct evaluation of the risk of aspiration [4-6]. However, there is a lack of studies evaluating swallowing function after ischemic stroke using cough and respiration function. Most of the studies investigating dysphagia and respiratory functions were related to myopathy such as amyotrophic lateral sclerosis. In this study, we focused on the correlation of cough and respiration functions with swallowing ability for the evaluation of patients with ischemic stroke.
In this study, we examined the correlation between videofluoroscopic dysphagia scale (VDS), peak cough flow (PCF), and pulmonary function test (PFT). Additionally, we investigated the role of PCF and PFT in evaluating dysphagia.
MATERIALS AND METHODS
Subjects
This study was targeted at patients hospitalized between March 2016 and February 2017 in Kosin University Gospel Hospital. Among the 213 patients who underwent videofluoroscopic swallowing study (VFSS) within 3 weeks of acute ischemic stroke onset during this period, 57 were selected based on the following inclusion and exclusion criteria. The inclusion criteria were (1) ischemic stroke, (2) evaluation using VFSS due to suspected dysphagia symptoms, (3) measurement of PFT using spirometry and PCF using a peak flow meter in addition to VFSS, (4) ability to understand VFSS, PFT, and PCF tests, and (5) measurement of spirometry and PCF with maximum effort. Exclusion criteria were (1) hemorrhagic lesion, (2) recurrent ischemic stroke, (3) complaints of dysphagia due to disease other than stroke, and (4) a history of cardiopulmonary disease.
Videofluoroscopic swallowing study and videofluoroscopic dysphagia scale
VFSS was performed by two rehabilitation physicians and assisted by radiation technologists. The VFSS diet included 2 mL and 5 mL of diluted barium, pudding, crushed banana, porridge and rice using a spoon, and water intake using a cup, in that order. A test was interrupted if a large amount of aspiration was observed or if the residual volume delayed aspiration during the VFSS. All test procedures were recorded as video clips. Two physiatrists analyzed the video clips and evaluated the VDS score. In this study, we used the version of VDS released by Han et al. [7] in 2008. It evaluates lip closure, bolus formation, mastication, apraxia, tongue-to-palate contact, premature bolus loss, oral transit time, triggering of pharyngeal swallow, vallecular residue, laryngeal elevation, pyriform sinus residue, coating of pharyngeal wall, pharyngeal transit time, and aspiration. VDS scores range from 0 to 100. The lower the score, the more normal is the swallowing function, and the higher the score, the more difficult it is for the patient to swallow.
Peak cough flow
The PCF test was conducted by a physician specializing in physical medicine and rehabilitation using a personal beast flow meter (Philips Respironics Inc., Murrysville, PA, USA). When testing VFSS, subjects wore masks and were seated. Subsequently, they were instructed to breathe in and out deeply two to three times and then cough as hard as they could. The maximum value of the three attempts was selected.
Pulmonary function test
PFT was conducted using spirometry prior to VFSS. It was performed by an experienced respiratory technician; the tidal volume (TV), total lung capacity (TLC), forced vital capacity (FVC), and forced expiratory volume in one second (FEV1) were measured.
Statistical analysis
Pearson correlation coefficient was calculated via analysis of each patient’s VDS, FVC, FEV1, and PCF values. The VDS values were evaluated via linear regression analysis of PFT results (VC, TV, FVC, FEV1), and PCF.
Using the mean and standard deviation of the VDS value, the patients in Group 1 displayed VDS values at least one standard deviation below the mean value. The patients in Group 2 had VDS values within one standard deviation of the mean. Group 3 included patients with VDS values more than one standard deviation above the mean value. A one-way ANOVA of PCF, FVC, and FEV1 among the three groups was performed. Any significant differences post-test were analyzed using the Scheffe test.
All targeted patients were divided into those diagnosed with aspiration pneumonia during hospitalization and a those who were diagnosed. The average values of VDS, FVC, FEV1, PCF, and age were compared between the two groups.
Statistical analyses were conducted using SPSS version 21.0 (IBM, Armonk, NY, USA) and the significance level was set at p<0.05.
RESULTS
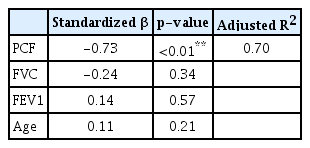
The correlation coefficients of VDS with PCF, FVC, and FEV1 were -0.84, -0.51, and -0.43, respectively, and were statistically significant at the a probability level of 0.01 (Table 1). A linear regression analysis identified PCF (standardized β coefficient=-0.73, p<0.01) as predictor of VDS (R2=0.70) (Table 2).
The value of PCF in 57 subjects was 133.86±84.64 (mean±standard deviation) and VDS was 33.00±26.46 (Table 3). The one-way ANOVA of VDS among groups divided by VDS score, PCF, FVC, FEV1, and age showed a statistically significant difference. In a post hoc study, PCF, FVC, and FEV values of groups 1 and 2 were higher than those of group 3. The age of groups 2 and 3 was higher than that of Group 1 (Table 3).
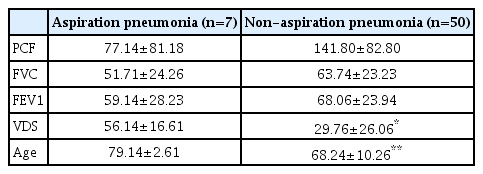
Among the 57 total patients, seven were diagnosed with aspiration pneumonia and the mean values of PCF, FVC, FEV1, VDS, and age in these patients were 77.14±81.18, 51.71±24.26, 59.14±28.23, 56.14±16.61, and 79.14±2.61, respectively, whereas the mean values of PCF, FVC, FEV1, VDS, and age in patients not diagnosed with aspiration pneumonia were 141.80±82.80, 63.74±23.23, 68.06±23.94, 29.76±26.06, and 68.24±10.26, respectively. The differences in VDS and age between groups of patients with aspiration pneumonia and non-aspiration pneumonia were statistically significant (p<0.05) (Table 4).
DISCUSSION
This study found that cough and respiratory ability, as assessed by PCF, FCV, and FEV1, were significantly correlated with dysphagia evaluation. Additionally, PCF was a statistically significant factor in dysphagia evaluation. Values of PCF, FCV, and FEV1 varied significantly among VDS groups, and PCF was significantly lower in patients diagnosed with aspiration pneumonia than non-aspiration pneumonia after ischemic stroke.
Decrease in lung volume is observed in the pulmonary function test of stroke patients, triggered by failure of neurologic control of the respiratory function, especially the cortico-diaphragmatic pathway, due to stroke lesions. It triggers a decrease in chest wall movement due to weakness or spasticity of the rib cage and abdominal muscle, and eventually results in a restrictive pattern of dyspnea [8,9].
Decreases in diaphragm movement and rib cage expansion in these stroke patients lead to decreased inspiratory reserve volume. This decrease is known to be the most important factor decreasing the vital capacity in stroke patients.
Types of cough are divided into voluntary cough, reflex cough, and expiration reflex. The voluntary cough flow rate is mainly used to evaluate the risk of aspiration pneumonia in patients suffering from a disease that represents dysphagia as stroke. Frequently, voluntary cough is measured via air flow, pressure, and expiratory muscle electromyography. In this study, air flow was measured using a bedside PCF meter [8,10].
In general, the swallowing process occurs during the expiration phase of the respiration process. Respiration stops during expiration, when the laryngeal and esophageal phase of the swallowing process occur, followed by the completion of the remaining expiration. In contrast, the voluntary cough and reflex cough mechanisms inspiration and pressure generating cough; therefore, the occurrence of cough during swallowing may be a factor implicated in intra-airway aspiration of food [7,10,11].
In contrast, in the case of laryngeal expiration reflex, the glottis is closed promptly when a solid, liquid or chemical irritant touches the vocal cord. The gas expulsion from the lungs occurs under strong pressure, resulting in cough. Preliminary inspiration does not occur during this expiration reflex. The laryngeal expiration reflex can be measured in patients inhaling 20% tartaric acid aerosol using a puffer; it is an invasive method, and therefore, is rarely used [7,11-13].
A review of studies investigating cough showed that the laryngeal expiration reflex reduce the risk of aspiration pneumonia in stroke patients and the intensity of voluntary cough shows a significant correlation with that of laryngeal expiration reflex [7,11].
The cough response during swallowing suggests stimulation of the mucosal water receptor in the upper larynx, that is, of food aspiration into the vocal cord. However, the laryngeal expiration reflex is a response that facilitates the discharge of aspirated food from the airway to the oral cavity, and plays a role in preventing pneumonia [7,11].
In this study, we found that the intensity of voluntary cough measured with a PCF meter was highly correlated with VDS in patients with ischemic stroke and was a statistically significant factor in evaluating dysphagia. In the study of Widdicombe et al. [14], voluntary cough was mostly associated with aspiration in dysphagia patients after stroke. Therefore, it is necessary to evaluate voluntary cough and conduct rehabilitation of stroke patients with dysphagia manifesting voluntary cough function. In addition, FVC and FEV1 also appeared to correlate with VDS and were used as measures of voluntary cough and expiratory muscle strength as well as PCF. However, their correlation coefficients were lower than that of PCF with VDS, and PCF showed a statistically significant difference between aspiration and non-aspiration groups. We focused specifically on the PCF value.
In studies of Kimura et al. [12], there was a significant difference in PCF between the groups of stroke patients with and without dysphagia. In addition, vital capacity and inspiratory reserve volume of the group of stroke patients with dysphagia were lower than in healthy controls. In this study, dysphagia was evaluated objectively using VDS. In addition, the association between cough and respiratory function, dysphagia, and aspiration pneumonia was assessed by correlation, linear regression analysis, and one-way ANOVA, and the mean values were compared according to the presence of aspiration pneumonia.
Treatment of dysphagia in patients with ischemic stroke entails an oral stage of chewing and laryngeal progression, a pharyngeal stage of improved laryngeal motion, and functional electrical stimulation [15,16]. The foregoing findings show that the improved ability to cough in patients complaining of dysphagia reduces the risk of aspiration pneumonia. Therefore, evaluation of respiratory and cough functions during the initial dysphagia evaluation and improved respiratory ability and cough via respiratory rehabilitation in general dysphagia treatment in patients complaining of dysphagia after ischemic stroke can ameliorate dysphagia and reduce the risk of aspiration pneumonia. The limitations of this study relate to the small number of target patients, and indistinguishable location of lesions.
In conclusion, PCF and respiratory function tests are indicated for the evaluation of swallowing function in patients diagnosed with ischemic stroke because of their association with the assessment of swallowing function and can facilitate the assessment of the risk of aspiration pneumonia.
Notes
No potential conflict of interest relevant to this article was reported.