Thoracic Outlet Syndrome Caused by Schwannoma of Brachial Plexus
Article information
Abstract
Schwannomas are benign, usually slow-growing tumors that originate from Schwann cells surrounding peripheral, cranial, or autonomic nerves. The most common form of these tumors is acoustic neuroma. Schwannomas of the brachial plexus are quite rare, and symptomatic schwannomas of the brachial plexus are even rarer. A 47-year-old woman presented with a 1-year history of dysesthesia, neuropathic pain, and mild weakness of the right upper limb. Results of physical examination and electrodiagnostic studies supported a diagnosis as thoracic outlet syndrome. Conservative treatment did not relieve her symptoms. After 9 months, a soft mass was found at the upper margin of the right clavicle. Magnetic resonance imaging showed a 3.0×1.8×1.7 cm ovoid mass between the inferior trunk and the anterior division of the brachial plexus. Surgical mass excision and biopsy were performed. Pathological findings revealed the presence of schwannoma. After schwannoma removal, the right hand weakness did not progress any further and neuropathic pain gradually reduced. However, dysesthesia at the right C8 and T1 dermatome did not improve.
INTRODUCTION
First described by Peet et al. [1] in 1956, thoracic outlet syndrome (TOS) involves compression of neurovascular structures between the base of the neck and the axilla. There are two main categories of TOS: the vascular form, which is caused by compression of the subclavian artery or vein, and the neurogenic form, which is caused by compression of the brachial plexus. The neurogenic form of TOS accounts for 95%-99% of all TOS cases [2].
Schwannomas are benign nerve sheath tumors composed of Schwann cells. Although schwannomas can occur in any part of the central or peripheral nervous system, they are most commonly found in the head, neck, and flexor surfaces of extremities. Schwannomas originating from the brachial plexus are rare, accounting for only approximately 5% of all cases [3]. At diagnosis, most schwannomas of the brachial plexus are asymptomatic [3,4]. In this article, we report the case of a patient with TOS caused by schwannoma of the brachial plexus.
CASE REPORT
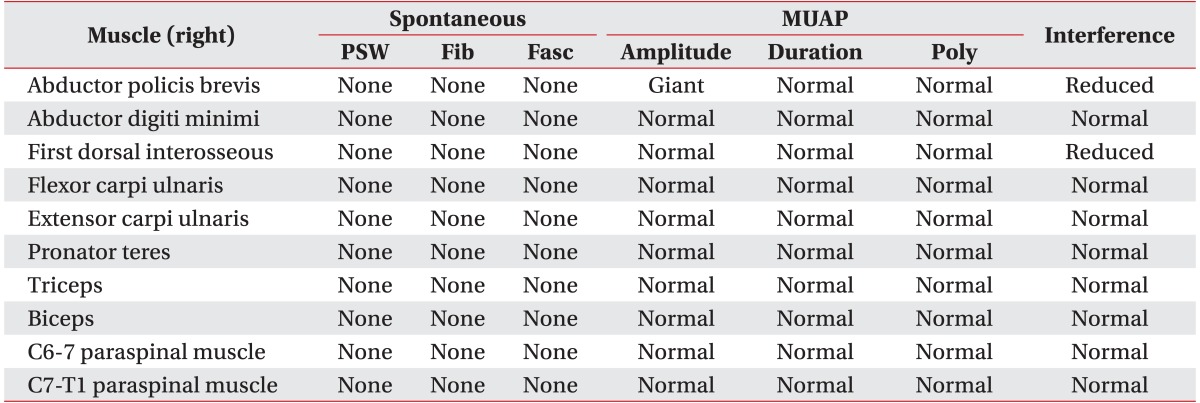
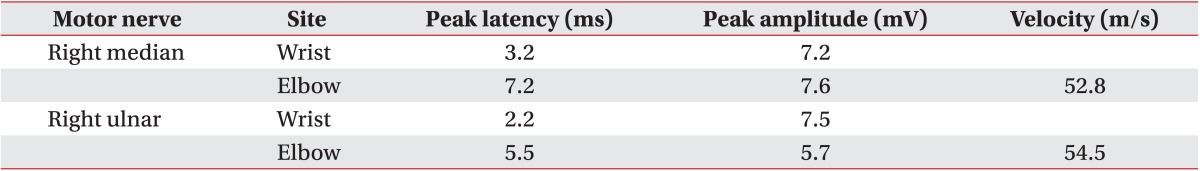
A 47-year-old woman visited the department of rehabilitation medicine complaining of pain, dysesthesia, and mild weakness in the intrinsic muscles of her right hand, each of which had persisted for approximately 1 year. The patient specifically reported dysesthesia and pain in her right upper arm, the medial part of her right forearm, and the fourth and fifth fingers of her right hand. There were no changes in color or pulse, and no indications of swelling or muscle atrophy. Plain cervical radiography and magnetic resonance imaging (MRI) were performed to rule out cervical or thoracic radiculopathy, but no specific abnormal findings were noted. Electrodiagnostic studies (EDS) showed that sensory and motor nerve conduction was normal. However, the F-wave study indicated slightly delayed onset latency at the right median and right ulnar nerves (right median, 27.8 ms; right ulnar, 27.8 ms). Needle electromyographic examinations of the right upper limb showed increased amplitude at the abductor pollicis brevis (APB) and reduced recruitment patterns at the APB and first dorsal interosseous (FDI). Results are presented in Tables 1-3. On physical examination, the Adson provocative test, hyperabduction maneuver, and elevated arm stress test were all positive. TOS was diagnosed. In the outpatient department, the patient received several forms of conservative management, including posture education, stretching exercises, trigger point injections, cervical stellate ganglion block, and medication. Despite this, her symptoms did not improve.
Nine months after receiving her first outpatient department treatment, the patient's symptoms were slightly aggravated. Motor power of the right finger intrinsic muscles was fair. The patient complained of a fixed and tender growing mass at the upper margins of her right clavicle. Ultrasonography revealed a 1.9×1.3×2.1-cm, well-marginated heterogeneous echoic mass at the lateral side of the right sternocleidomastoid muscle (Fig. 1). As ultrasonographic findings were strongly suggestive of a tumor, we performed enhancing right brachial plexus MRI, which revealed a 3.0×1.8×1.7-cm, well-marginated peripheral enhancing ovoid mass between the inferior trunk and the anterior division of the brachial plexus. T1-weighted images showed heterogeneous low signal intensity and T2-weighted images showed generally high signal intensity, with partial low signal intensity (Fig. 2).
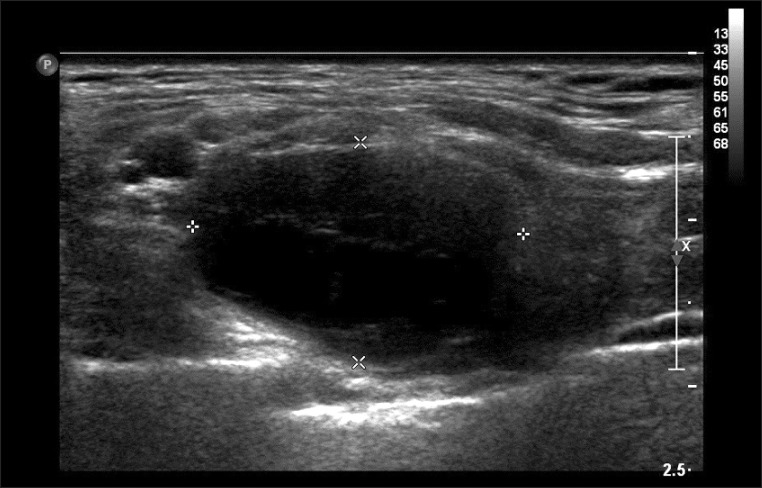
A well-defined heterogeneous echoic mass is apparent in the right lower anterior neck. The dimensions were 1.9×1.3×2.1 cm. The deep soft mass was located in the lateral margin of the sternocleidomastoid muscle.
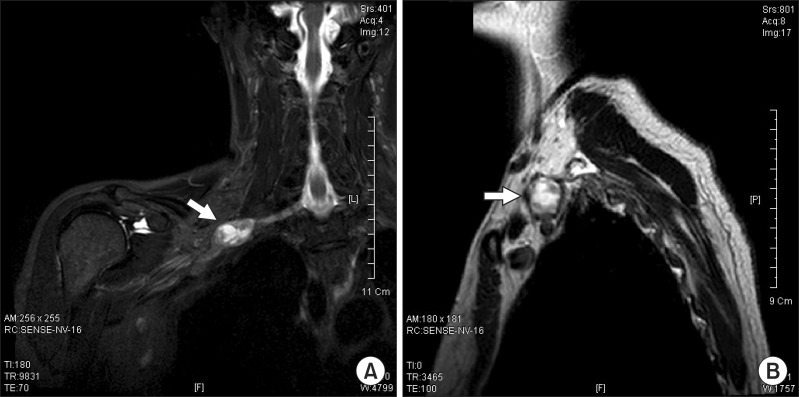
(A) T2 coronal magnetic resonance imaging (MRI) and (B) T2 sagittal MRI right brachial T2 coronal view images indicates an approximately 3.0×1.8×1.7 cm ovoid mass (arrow) between the inferior trunk and the anterior division of the brachial plexus (A). At the lateral arc of the right first rib, the T2 sagittal view also revealed an ovoid mass (arrow).
Subsequently, the patient was referred to the orthopedic department, where she underwent surgical mass removal. A biopsy was performed during surgery. On microscopic examination, hematoxylin and eosin staining revealed a strongly stained spindle cell neoplasm displaying Antoni A and Antoni B areas with Verocay bodies, which are characteristic findings of schwannoma (Fig. 3). After removal of the schwannoma, the weakness in the patient's right hand did not progress any further and her neuropathic pain gradually reduced. However, dysesthesia at the right C8 and T1 dermatome did not improve.
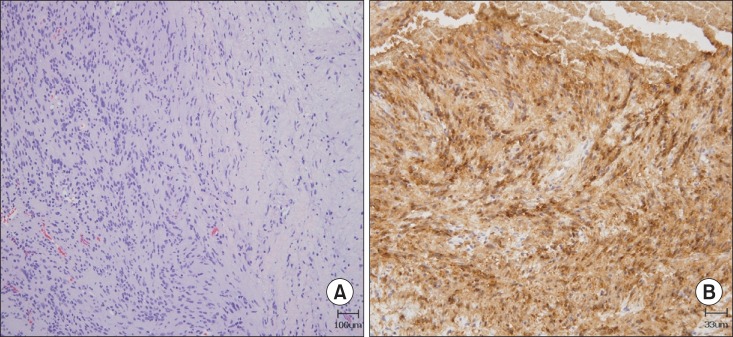
(A) Hematoxylin and eosin-stained tumor composed of spindle-shaped neoplastic Schwann cells with alternating areas of compact, elongated cells with occasional nuclear palisading (Antoni A region on the left field) and less cellular, loosely textured areas (Antoni B region on the right field) (×100). (B) Immunohistochemical staining (×300 magnification) demonstrated that the tumor cells were positive for S-100 protein.
Twenty-eight months after surgery, the patient underwent follow-up EDS. Needle electromyographic examinations showed increased amplitude and reduced recruitment patterns at the right APB, FDI, and abductor digiti minimi. Motor nerve conduction study showed that compound muscle action potential (CMAP) amplitudes of the right median and ulnar nerves were increased as compared with the previous test results (right median, 10.9 mV; right ulnar, 11.0 mV). The F-wave study for the right median and ulnar nerves indicated normal onset latency (right median, 24.8 ms; right ulnar, 26.7 ms).
DISCUSSION
TOS is a complicated entity characterized by various symptoms and signs of the upper limb. Each of these signs and symptoms arises from compression of neurovascular structures between the base of the neck and axilla. The underlying cause of the compression itself varies from case to case. Causes include physical trauma, poor posture, repetitive activity, tumor, and anatomical defects, such as first rib fixation and cervical rib [2,5]. Since there are no standardized objective tests, TOS is difficult to diagnose. The clinician must rely on patient history and positive findings during physical examination. Initially, patients with TOS should be treated with conservative methods, such as behavior modifications, stretching exercise, physical therapy, medications, and injection therapy [2,5,6]. Most patients respond to conservative management and do not require surgical intervention. If a patient's symptoms do not improve after 3 months of conservative management, surgical treatment should be considered [7].
Although schwannoma is the most common neurogenic tumor of the thorax, schwannoma of the brachial plexus is quite rare. Most schwannomas of the brachial plexus are incidental tumors [4]. Only a few cases of brachial plexus schwannoma have been reported in Korea. In two cases, the patients' chief complaint was a palpable mass without any clinical symptoms [8,9]. In another case, the patient complained of radiating pain of the upper limb with a palpable mass [10]. Indeed, none of the patients presented TOS signs and symptoms without a palpable mass. To our knowledge, this is the first case report of TOS caused by non-palpable schwannoma of the brachial plexus in Korea.
It is difficult to diagnose tumors of the brachial plexus in patients with pain of the upper limb and dysesthesia. These tumors may be difficult to palpate because they are usually located in deep tissue and/or are quite small. Clinical symptoms, physical examination, EDS, brachial plexus ultrasonography, and MRI could all help the clinician successfully diagnose symptomatic tumors of the brachial plexus. Of these tests, brachial plexus MRI is the most valuable imaging study that can provide information about the tumor's anatomy, size, origin, and surrounding structures [2].
In the diagnosis of this case, we only performed EDS on the right upper limb, not on both upper limbs. As EDS results corresponded to C8 and T1 radiculopathy more closely than brachial plexopathy, TOS of an unknown cause was diagnosed. Due to this diagnosis, surgical therapy was delayed. In retrospect, it would have been better to conduct EDS on both upper limbs. We also should have compared the CMAP amplitudes of both upper limbs. Finally, early brachial plexus MRI should have been performed, which would have helped discover any brachial plexus lesion.
Twenty-eight months after surgery, follow-up EDS for the right median and ulnar nerves showed improvements in F-wave onset latency and CMAP amplitude. Although neuropathic pain of right upper limb was improved, the patient still complained of weakness in the right finger intrinsic muscles and dysesthesia in the right C8 and T1 dermatome. Approximately 21 months passed between the onset of the patient's symptoms and surgical intervention. Therefore, we believe that the patient's persistent weakness and dysesthesia following surgery could have been avoided by more timely surgical intervention.
Our experience with this patient suggests that neurogenic tumors of the brachial plexus should be suspected, when patients with TOS do not respond to conservative management for a long time. We emphasize that brachial plexus MRI can help the clinician achieve accurate and rapid diagnosis of neurogenic tumors of the brachial plexus, and early surgery is the treatment of choice.
Notes
No potential conflict of interest relevant to this article was reported.