The Effects of Visual and Haptic Vertical Stimulation on Standing Balance in Stroke Patients
Article information
Abstract
Objective
To explore the effect of visual and haptic vertical stimulation on standing balance in post-stroke patients.
Methods
Twenty-five post-stroke patients were recruited. We measured left/right standing pressure differences and the center of pressure (COP) parameters for each patient under three different conditions: no stimulation, visual, and haptic stimulated conditions. First, patients stood on a posturography platform with their eyes blindfolded. After a rest period, the patients stood on the same platform with their eyes fixed to a 1.5-m luminous rod, which was placed at a vertical position in front of the patients. After another rest period, the patients again stood touching a vertically placed long rod in their non-hemiplegic hand with their eyes blindfolded. We collected the signals from the feet in each condition and obtained the balance indices.
Results
Compared with the no stimulation condition, significant improvements were observed for most of the COP parameters including COP area, length, and velocity for both the visual and haptic vertical stimulation conditions (p<0.01). Additionally, when we compared visual and haptic vertical stimulation, visual vertical stimulation was superior to haptic stimulation for all COP parameters (p<0.01). Left/right standing pressure differences, increased, although patients bore more weight on their paretic side when vertical stimulation was applied (p>0.01).
Conclusion
Both visual and haptic vertical stimulation improved standing steadiness of post-stroke patients. Notably, visual vertical stimulation was more effective than haptic stimulation.
INTRODUCTION
Standing balance can be affected in several ways after stroke, including joint motion limitation, motor weakness, altered muscle tone, sensory deficits, and cognitive problems [1-3]. In addition, spatial cognitive dysfunction, particularly misperception of verticality, can impair standing balance control, and its importance is increasing [4,5]. Some reports have considered upright structures in visual surroundings or a vertically placed touchable rigid bar [6,7]. Unfortunately, correct visual stimulations were not provided, and the goals of these studies were to analyze the effects of additional sensory input. In our study, we applied the correct visual vertical (VV) and haptic vertical (HV) stimulation to adjust for misperception of verticality. Few studies have attempted a direct therapeutic correction of the misperception of verticality, an important cause of imbalance after stoke. Accordingly, we investigated the direct effect of VV and HV stimulation on standing balance in post-stroke patients.
MATERIALS AND METHODS
Subjects
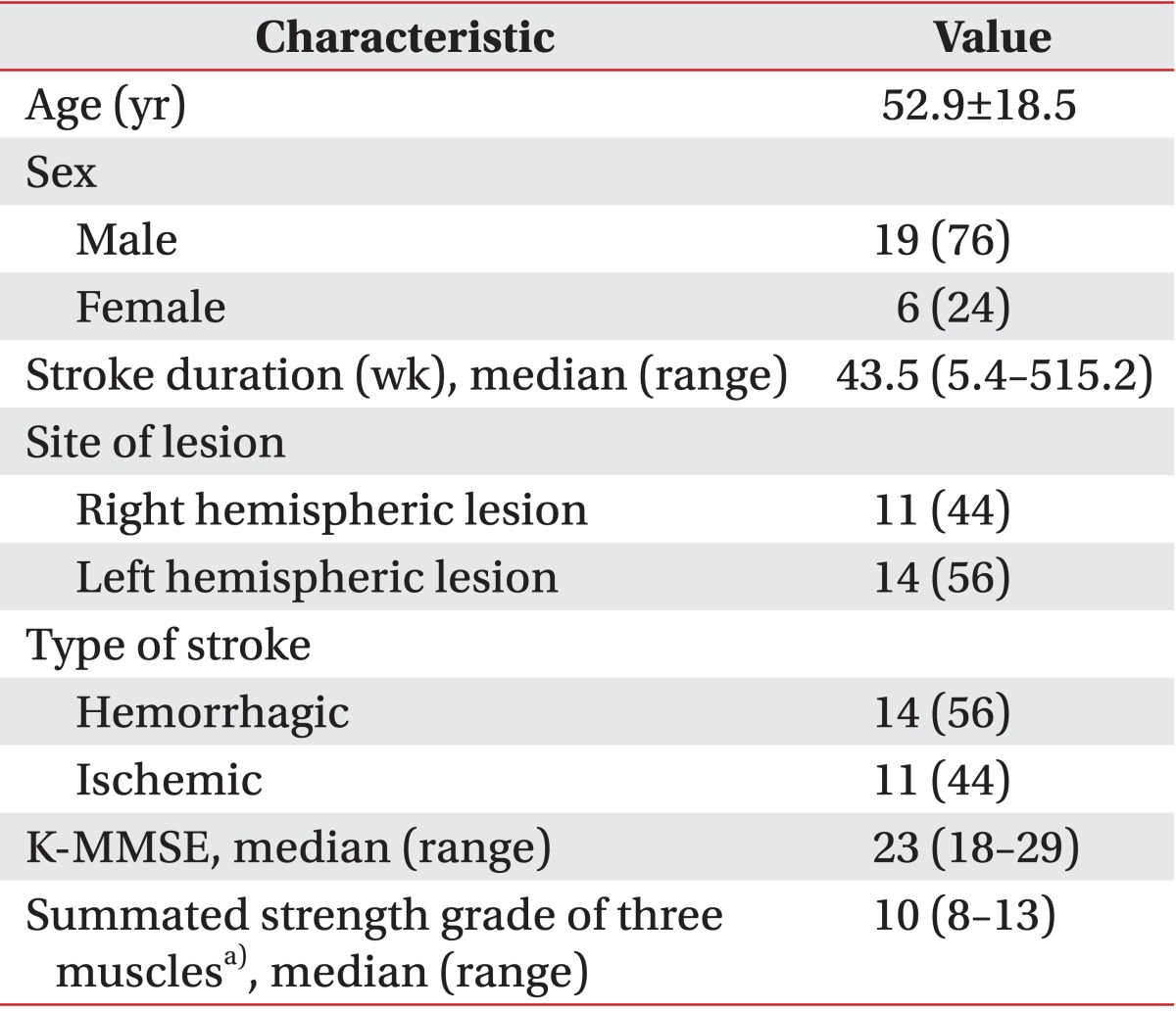
Twenty-five post-stroke patients participated in this study. They received balance training at our rehabilitation center between February and March 2011. They all had difficulties with standing balance with hypoesthesia on the contralesional side. We performed a complete neurologic examination. Cognition was evaluated through the Korean version of the Mini-Mental State Examination (K-MMSE). Motor weakness of the hip, knee, and ankle extensors was evaluated by the Manual Muscle Strength Test. We summed the grade of each of the muscle strength values. Motor weakness ranged from 0 (no contraction) to 15 (normal strength). Hypoesthesia of the paretic side was assessed through the Semmes-Weinstein monofilament. We pressed the pulp of the big toe until the monofilament buckled. If the patient had no sensation or a reduced sensation compared to the contralateral side, it was considered hypoesthesia. An ear, nose, and throat examination was conducted to rule out the presence of a vestibular disorder. All patients were able to stand for at least 60 seconds without technical or human aid. A patient was excluded if he or she had visual problems, a vestibular dysfunction, prior lower extremity surgical history, degenerative disease, or comprehension problems that might affect balance. Approval for the study was obtained from the Medical Ethics Committee of our institution. All patients gave written informed consent in accordance with the guidelines of the ethics committee.
Methods
Evaluation tool
Postural body sway was measured using a posturography in a silent room. We used BioRescue (RM Ingenierie, Rodez, France), which includes a platform (610 mm × 580 mm × 10 mm), equipped with 1,600 pressure sensors ensuring precise analysis, software, and a monitor. BioRescue shows the center of pressure (COP) trajectory and measures its sway length (cm), area (mm2), and mean velocity (cm/s). In addition, BioRescue yields direct information about the symmetry of weight-bearing by the measuring left/right standing pressure differences. A larger sway measurement or a left/right standing pressure difference indicated poorer standing balance.
Measurement
We analyzed the COP sway measures and left/right standing pressure differences under three different conditions: 1) no vertical stimulation, 2) VV stimulation, or 3) HV stimulation. The patients were instructed to sway as little as possible during the three trials, and the trials were separated by seated rest periods. At first, under the no vertical stimulation condition, the patients stood barefoot for 60 seconds with one foot on each of two platforms with their eyes blindfolded while maintaining their body in a neutral position (i.e., heels 9 cm apart, feet 30° rotated externally, and arms hanging freely along the body in the most comfortable position).
After a 5-minute rest period to give the participants a correct VV perception, we used a vertically placed 1.5 m long luminous rod. The patients were instructed to stand exactly as described in the paragraph above. They were instructed to keep their eyes forward. The rod was located directly in front of the patients. After another 5 minutes rest period to allow the patients to obtain the correct HV perception, the patients stood again while gently touching a vertically placed long rod in their non-hemiplegic hand; they were blindfolded. To minimize the mechanical effect provided by the transient forces developed between the hand and the rod, the subjects were instructed to gently touch the rod using their finger tips. We collected signals from both feet and obtained balance indices under each condition. We started to measure the balance indices after confirming the patient's postural stabilization to exclude the initial stabilization period. A 5-minute rest period was allowed between each of the tests. Patients received a sufficient explanation of the test prior to their participation.
Statistical analysis
Left/right standing pressure difference analysis was performed by paired t-tests and the data are presented as mean±standard deviation. The distributions of COP sway length, area, and mean velocities were negatively skewed; hence, these data were analyzed by Wilcoxon signed-rank test. Potential confounding factors included hemiplegic side, stroke type, stroke duration, the K-MMSE, and motor weakness. The associations between the hemiplegic side and the stroke type with the balance indices were analyzed by t-test and Wilcoxon signed-rank test. The associations between duration, the K-MMSE, and motor weakness with the balance indices were analyzed by Spearman correlations. The alpha level was set at 0.01. All analyses were performed using SAS ver. 9.2 software (SAS Institute, Cary, NC, USA).
RESULTS
General characteristics of the subjects
Twenty-five hemiparetic patients (nineteen men and six women) participated in this study. The median age was 52.9±18.5 years, and the time since their stroke was 43.5 weeks (range, 5.4-515.2 weeks). All brain lesions were identified through the use of magnetic resonance imaging or computed tomography. Fourteen patients had a brain lesion in their left hemisphere, whereas 11 had right-sided lesions. The stroke was hemorrhagic in 14 patients and ischemic in 11. The median K-MMSE score was 23 points (range, 18-29 points). The median summed strength grade of the three muscles was 10 (range, 8-13). The characteristics of the subjects are displayed in Table 1.
COP sway area
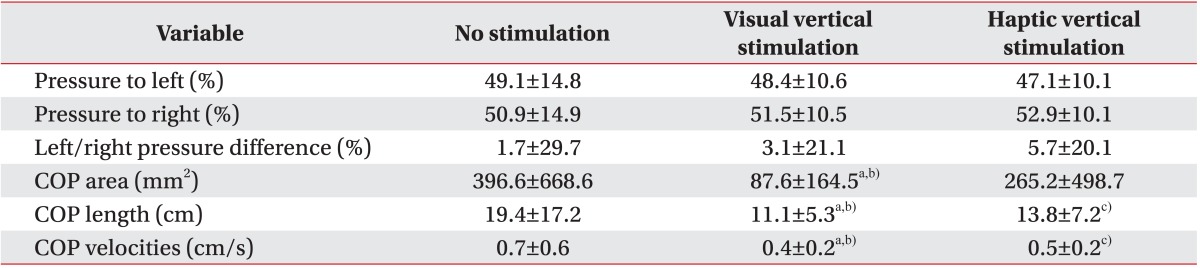
The COP sway areas were reduced under both the VV and the HV stimulation conditions compared with those under the no stimulation condition (no stimulation, 396.6±668.6 mm2; VV stimulation, 87.6±164.5 mm2; HV stimulation, 265.2±498.7 mm2) (Table 2). A significant difference was shown in the VV stimulation condition, compared to the no stimulation condition (p<0.001); no significant difference was observed in the HV stimulation condition compared to the no stimulation condition (p=0.05) (Table 2). Additionally, visual stimulation was statistically superior to haptic stimulation (p=0.004) (Table 2).
COP sway length
Both VV and HV stimulation produced statistically significant reductions in COP sway lengths compared to that in the no stimulation condition (p<0.001 in the VV condition and p=0.003 in the HV stimulation condition) (Table 2). Notably, marked reductions were shown under the VV stimulation condition compared with the HV stimulation condition (p=0.001) (Table 2).
COP sway mean velocity
Similar to COP sway length, both stimulations reduced COP sway mean velocities compared to the no stimulation condition (p<0.001 in the VV stimulation condition and p=0.004 in the HV stimulation condition) (Table 2). VV stimulation had a greater effect on the COP sway mean velocities than HV simulation (p<0.001) (Table 2).
Left/right standing pressure difference
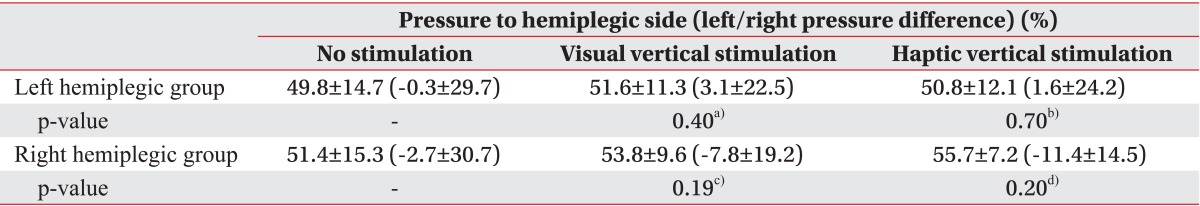
Patients bore more weight on the right side regardless of their hemiplegic side (50.2%±15.0% in the left hemiplegic group and 51.4%±15.3% in the right hemiplegic group) (Tables 3, 4). After the VV and the HV stimulation, patients showed a trend of bearing more weight on their paretic side compared to the no stimulation condition-left hemiplegic group: 51.6%±11.3% (p=0.40) in the VV stimulation condition, 50.8%±12.1% (p=0.70) in the HV stimulation condition; right hemiplegic group: 53.8%±9.6% (p=0.19) in the VV stimulation condition, 55.7%±7.2% (p=0.20) in the HV stimulation condition (Table 3). However, unlike the COP sway parameters, left/right standing pressure differences increased when VV or HV stimulation was applied (no stimulation 1.7%±29.7%; VV stimulation 3.1%±21.1%; HV stimulation 5.7%±20.1%) (Table 2). The amount of weight-bearing asymmetry was not statistically significant compared to the no stimulation condition (p=0.64 in the VV stimulation condition and p=0.35 in the HV stimulation condition) (Table 2).
Association between hemiplegic side, stroke type, and balance indices
No associations were detected between the hemiplegic side (left or right) and stroke type (infarction or hemorrhage) and the stroke indices (all p>0.01) (Table 4).
Associations between stroke duration, cognition, muscle weakness, and balance indices
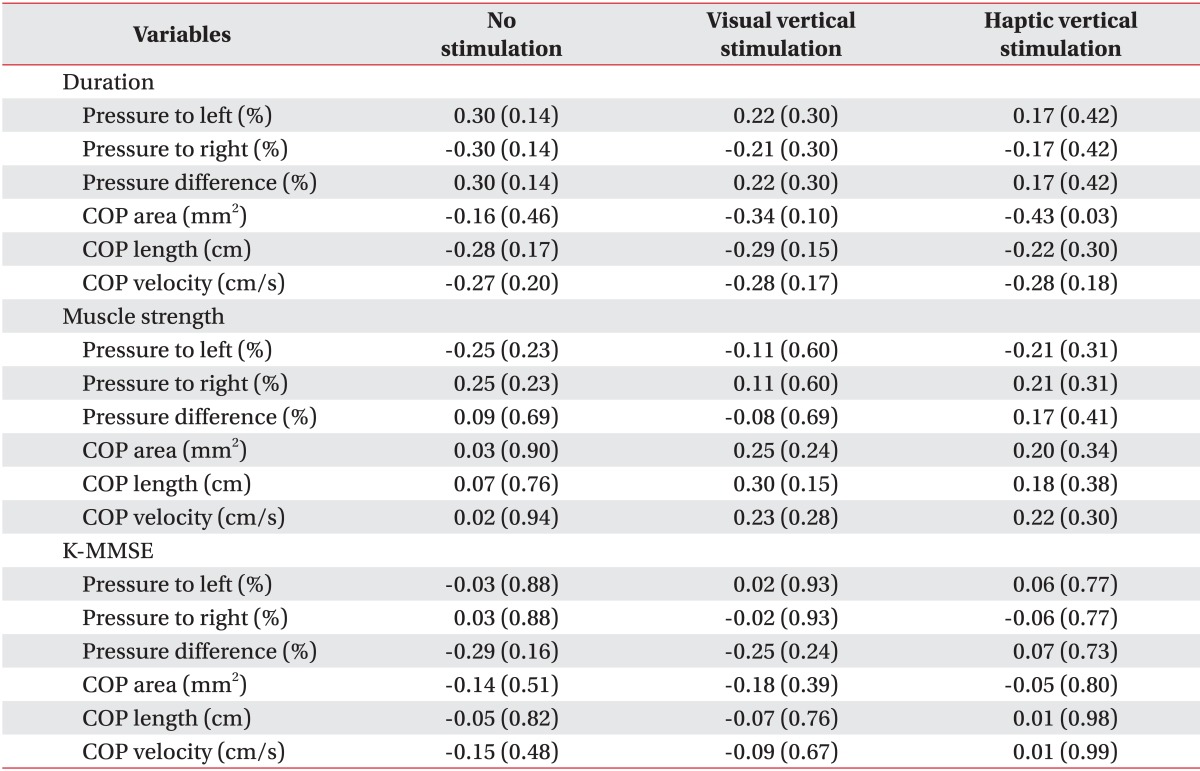
No associations were detected between stroke duration, cognition, and muscle weakness and the balance indices (all p>0.01) (Table 5).
DISCUSSION
Many attempts have been made to provide compensatory sensory stimulation including transcutaneous electrical nerve stimulation [8], functional electrical stimulation [9], electromyographic feedback [10] or force feedback training to improve standing balance [11]. Unfortunately, none of the physiotherapies are superior for promoting balance recovery in stroke patients [12]. Thus, a novel rehabilitation approach is needed.
Here, we targeted the misperception of verticality, a major cause of impaired standing balance. The correct perception of verticality is formed by integrating adequate visual, vestibular, and somatosensory input [13]. Based on these afferent inputs, internal sensory integration process construct perception of verticality, which is normally parallel to the earth's vertical axis [13,14]. It can be evaluated through different modalities, such as visual, haptic, and postural [13,15]. Among these, we targeted visual and haptic verticals. Separate 867 exist for the perception of VV and HV [4,16]. VV perception is mainly based on visual and vestibular input from peripheral organs [4]. In contrast, HV perception originates from peripheral somatosensory input [4,16]. There is often dissociation between the two vertical stimulations [4,16]. Nevertheless, post-stroke patients could have a problem with afferent input or integration disorders of different sensory modalities that may lead to the misperception of verticality [14]. In our study, all subjects had somatosensory loss on the contralesional side. We postulated that our patients might have a misperception of verticality based on the abovementioned reasons; thus, we tried to reduce this bias through the appropriate manipulation, such as correct vertical stimulation. In general, to assess the visual vertical, the subject was instructed to adjust a luminous rod in the vertical direction in the darkness [17,18]. The subject was asked to set a rotating bar to the vertical direction by using his/her tactile sense in the darkness to evaluate the haptic vertical [17,18]. Based on these evaluation methods, we designed our study to provide the correct VV and HV stimulations; we analyzed their effects through posturography. The typical characteristics of unstable standing in post-stroke patients are weight-bearing asymmetry with more weight placed on the nonparetic leg and a large postural sway [19]. Considering these characteristics, we focused on two aspects of standing balance: steadiness and symmetry. Steadiness is the ability to maintain a standing posture with minimal movement (i.e., sway) [20]. It is generally assessed as the amount of COP displacement [21,22]. COP sway length, area, and velocity are commonly used to assess postural control [21] and were used in the present study as parameters of COP displacement. However, symmetry is a term used to describe an equal weight distribution between the two feet in a standing position [20]. It can be assessed by measuring the left/right pressure difference. Achieving weight-bearing symmetry with minimal sway has been viewed as a primary goal of rehabilitation [23].
In our study, the COP sway parameters were reduced in both the VV and the HV stimulation conditions. That is, subjects were more stable when gravitational cues were provided. It has been proposed that gravitational cues are favored during orientation processing because they specify the gravitational vertical, which is used as a reference [24]. Although the mechanism underlying this effect has not been characterized, it may be due to adapting the excitability of the integrative centers and the brain circuits implicated in balance control [25]. Additionally, a greater reduction in COP parameters was observed in the VV stimulation condition than the HV stimulation condition. When surrounded by many visual, vestibular, and somatosensory stimuli, stroke patients may favor one system over another to control standing balance [26]. It has also been reported that post-stroke patients depend more on visual information for balance control than healthy age-matched groups [23]. It has long been known that vision is a major determinant of balance control [18] and excessive reliance on visual input may be a learned compensatory response that occurs over time, particularly in patients who have a somatosensory impairment [26]. Our patients likely compensated for their impaired balance using surrounding visual information rather than information from other sensory modalities. This would explain the observation of greater improvements in the VV stimulation condition. However, no reports have indicated that visual stimulation is superior to haptic stimulation for standing balance. Additional research is needed to compare the effect of different sensory modalities, which often have dissociable effects, on vertical perception [13,16,27].
Interestingly, unlike the left hemiplegic patients, the right hemiplegic patients bore more weight on their weaker side. This result is inconsistent with the general concept that more weight is loaded on the nonparetic side in hemiplegic patients [19]. However, according to one report, more weight-bearing on the paretic side is observed in about 12% of stroke patients [28]. In that study, no individual determinants of the weight-bearing side were found. Similarly, we investigated sex, age, stroke duration, stroke type, cognition, and muscle power and found no individual determinants. This may be due to sustained pushing behavior or a compensatory strategy learned in a rehabilitation program [28]. More weight on the paretic side has an advantage in that it allows for the rapid step of the intact limb in case of instability [28]. Additionally, we found that more weight was shifted to the paretic side and weight-bearing asymmetry deteriorated under the VV and HV stimulation conditions, although significant differences were not observed. However, weight-bearing asymmetry may not be related with COP parameters [29] and it is not clear whether weight-bearing asymmetry is associated with postural instability [30]. Rather it is effective compensatory method to restore standing balance after stroke [31]. In our study, COP parameters were improved despite more weight-bearing to the paretic side and exaggerated weight-bearing asymmetry. This may have been due to increasing the somatosensory input and enhancing kinetic contribution from paretic limb. Our patients had sufficient power in their paretic limb to tolerate more weight.
There are some limitations in interpreting and verifying the results of this intervention. First, the number of subjects in the study was too small to generalize the results to a wider group of people with stroke. Second, we did not assess the existence of visual and haptic vertical misperception of subjects. Instead, we assumed that none of the subjects had a correct perception of verticality because they all had contralesional somatosensory deficits. Although the degree of hypoesthesia can be a factor that affects verticality perception, we only determined whether the patients had hypoesthesia. Third, when alternating between the three intervention approaches (i.e., no stimulation, VV stimulation, and HV stimulation), the carryover effect from one approach to another was not strictly controlled. Fourth, no statistical analysis was conducted for the interaction between brain lesion and stimulation. The central vestibular pathways (e.g., the brainstem, thalamus, cortex) of both cerebral hemisphere, sensory pathways (e.g., the thalamus, sensory cortex), and regions implicated in visuospatial process (e.g., the parietal cortex) are known to be associated with verticality perception [10]. Because the sample was small and the patients' brain lesions were heterogeneous, we could not determine whether an interaction existed. Fifth, two types of information were given to the patients when the HV stimulation was applied. One was related to the provision of a vertically fixed reference point in space and the other was related to the information provided by transient mechanical forces developed between the hand and the contact surface. We intended to provide the first type of information; the latter type of information was unintended. To minimize this effect, subjects were instructed to gently touch the vertical rod using their finger tips. Unfortunately, we cannot rule out an effect of the latter type of information.
In conclusion, we focused on the effect of direct visual and haptic vertical stimulation on standing balance of stroke patients. Left/right pressure difference, namely, weight-bearing symmetry got worse with either stimulation. However, both stimulations reduced COP displacement parameters. In other words, standing balance, particularly steadiness, improved by providing the correct vertical stimulation. Furthermore, this effect was more prominent in VV stimulation than HV stimulation. Thus, this approach may provide the basis for an effective rehabilitation program for post-stroke patients who suffer from standing balance impairment.
ACKNOWLEDGMENTS
This work was supported by the Institute of Clinical Medicine Research of Bucheon St. Mary's Hospital, Research Fund, BCMC 10AH08. The statistical consultation was supported by the Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), Ministry of Health & Welfare, Republic of Korea.
Notes
No potential conflict of interest relevant to this article was reported.