The Effect of Continuous Epidural Electrical Stimulation on Neuronal Proliferation in Cerebral Ischemic Rats
Article information
Abstract
Objective
To investigate the effect of electrical stimulation (ES) on the recovery of motor skill and neuronal cell proliferation.
Methods
The male Sprague-Dawley rats were implanted with an epidural electrode over the peri-ischemic area after photothrombotic stroke in the dominant sensorimotor cortex. All rats were randomly assigned into the ES group and control group. The behavioral test of a single pellet reaching task (SPRT) and neurological examinations including the Schabitz's photothrombotic neurological score and the Menzies test were conducted for 2 weeks. After 14 days, coronal sections were obtained and immunostained for neuronal cell differentiation markers including bromodeoxyuridine (BrdU), neuron-specific nuclear protein (NeuN), and doublecortin (DCX).
Results
On the SPRT, the motor function in paralytic forelimbs of the ES group was significantly improved. There were no significant differences in neurological examinations and neuronal cell differentiation markers except for the significantly increased number of DCX+ cells in the corpus callosum of the ES group (p<0.05). But in the ES group, the number of NeuN+ cells in the ischemic cortex and the number of NeuN+ cells and DCX+ cells in the ischemic striatum tended to increase. In the ES group, NeuN+ cells in the ischemic hemisphere and DCX+ cells and BrdU+ cells in the opposite hemisphere tended to increase compared to those in the contralateral.
Conclusion
The continuous epidural ES of the ischemic sensorimotor cortex induced a significant improvement in the motor function and tended to increase neural cell proliferation in the ischemic hemisphere and the neural regeneration in the opposite hemisphere.
INTRODUCTION
After malignant neoplasms, cerebrovascular disease has become the second leading cause of death in the past ten years [1]; hence, clinical research studies of this disease have become more important than ever before. Till now, many interventional procedures for stroke have been suggested including conservative treatment, surgical treatment, and rehabilitation treatment. Electrical stimulation (ES) treatment of the cerebral motor cortex, which has been employed to control intractable epileptic seizures or central pain in patients with motor paralysis, has been shown to induce improvement of motor function [2]; subsequent recovery of arm motor function was reported after conducting the ES treatment of the cerebral cortex in chronic hemiplegic stroke patients with severe impairment. Therefore, it was suggested that ES of the cerebral cortex affects neural plasticity of the brain [3,4].
The mechanisms for motor function recovery through ES have been explained previously where it has been shown that ES reduces neural excitotoxicity [5], protects the nerves by increasing γ-aminobutyric acid levels in the ischemic cerebral cortex and promoting the regrowth of axons [6] and increases neurogenesis or neural cell division [7,8]. Especially, it is known that ES treatment of the cerebral cortex contributes to enhancing synaptic plasticity by increasing dendritic densities and synaptogenesis in the brain [9-11]. In a study of the markers of structural remodeling of the brain using epidural ES in a cerebral ischemic rat model, continuous ES treatment produced a positive outcome of synaptogenesis along with an increase in axonal sprouting and neural plasticity of dendrites in the ischemic penumbra [12]. Despite the fact that there are studies confirming some effects of ES treatment, however, studies are still unable to explain the mechanism for the effect of ES treatment on neural differentiation and neurogenesis. Moreover, it is quite rare to find neurochemical studies of immunohistochemical (IHC) changes in neural stem cells and cerebral neurogenesis following ES.
Therefore, the authors aimed to investigate the effect of ES treatment on neuronal differentiation and proliferation in the ischemic penumbra and nearby cerebral tissues by assessing the neuronal markers after continuous application of epidural ES treatment in a rat model of photochemically induced photothrombotic focal ischemia.
MATERIALS AND METHODS
Subjects
Twenty-eight male Sprague-Dawley rats (Samtako Bio Korea, Osan, Korea) weighing between 250 to 300 g were enrolled. All study subjects were subjected to photothrombotic cerebral ischemia, and an epidural electrostimulator was placed in the sensorimotor cerebral cortex; subsequently, behavioral and neurologic examinations were conducted for the following two weeks. Study subjects were randomly divided into two groups; the ES group was composed of 15 rats with continuous application of ES, while the control group was composed of 13 rats without application of ES. This study was approved by the Institutional Animal Care and Use Committee of Wonkwang University College of Medicine.
Methods
Induction of photothrombotic cerebral ischemia
Intramuscular anesthesia with ketamine hydrochloride (60 mg/kg) and xylazine hydrochloride (7 mg/kg) was administered to the subjects. Stereotaxic apparatus (Model 900 Small Animal Stereotaxic; David Kopf Instruments, Tujunga, CA, USA) was used to fix the head of the subject in a prone position. The exposed skull of the dominant hemisphere was irradiated with white light at 1.0 W/cm2 (illuminator-halogen, FOK-150X/150R; Fiber Optic Korea Co., Cheonan, Korea). Terminal irradiation was applied using a 4-mm diameter optical fiber that was accurately placed at a point 3 mm to the lateral side and 1.5 mm anterior to the bregma such that the optical fiber was placed over the primary motor cortex based on the rat brain map [12,13]. To induce a response to photochemical thrombotic cerebral ischemia, 20 mg/kg of Rose Bengal (Sigma-Aldrich Co, St. Louis, MO, USA) was slowly injected for two minutes into the femoral vein after two minutes of initiating irradiation, and irradiation followed by skin suturing for hemostasis was continued for another 16 minutes [14]. Since this photothrombotic cerebral ischemia model allows for development of the intended brain infarct of an adequate size and type, it has a high reproducibility and the etiology is similar to that of human cerebral ischemia [14].
Electrode implantation for ES [12,15]
Craniectomy measuring 0.4×0.2 cm in size was performed over the region that was partly overlapping with the sensorimotor cerebral cortex of the dominant hemisphere, and a monopolar ES electrode was placed outside the dura mater. A reference electrode that was connected to this monopolar electrode by wires was screwed into the occipital bone, and the scalp was sutured after fixation with bone cement. The socket that was inserted into the implanted electrode was connected to the electrostimulator with wires passing through the swivel. While ES was continuously administered, the behavior-stimulation box that can perform simultaneous behavioral assessment was used when necessary [12,15]. In the ES group, ES was continuously administered for 24 hours a day, for 2 weeks. Anodal high frequency (50 Hz, 220 µs) electrostimulation was applied, and the intensity of ES was based on the movement threshold [15]. The movement threshold was defined as the minimum intensity of ES that can produce a movement of the facial muscles, whiskers and forelimbs opposite to the lesion, and it was increased sequentially by 0.1 V every 30 seconds after being initiated at 0.8 V [16]. In this study, 50% of the intensity of the movement threshold was selected as the intensity of continuous ES [12,15]. When a provocative response was not detected on applying ES greater than 5 V, normality of the wire and electrode was investigated after sacrificing the subject [15]. All conditions applied to the control group were identical to those applied to the ES group except for ES.
Behavioral assessment after induction of cerebral ischemia (single pellet reaching task)
Rats went through the adaptation process with daily behavioral training for 2 weeks prior to the experiment in order to complete the reaching task, in which they were to acquire the pellets (Bioserve, Beltsville, MD, USA) by extending their dominant forelimb through a long slit with a width of 1×15 cm in the front of the behavior-stimulation box. Single pellet reaching task (SPRT) assessed the performance of the rats by analyzing whether the rats grasp the pellet that is placed on the feed bucket with their dominant forepaw and accurately put it into their mouth [17]. Success rate of SPRT was calculated as percentage after the rats performed the reaching task 20 times. SPRT was conducted in all study subjects at the same time every day from 5 days prior to the surgery for inducing cerebral ischemia to 2 weeks after the surgery.
Neurologic examination after induction of cerebral ischemia
Two types of neurologic examinations were conducted by an examiner, who was unaware of the experimental group (blinded test), at the same time every three days after the surgery. Schabitz et al. [18] examination is mostly employed in studies using the photothrombotic stroke model, and it comprises of motor function assessment, observation while laying the rat on the floor, and the observation of reflex and abnormal behaviors; the results of this examination are evaluated on a 1 through 10 scale. Menzies et al. [19] examination was developed for application in a rat model of cerebral artery occlusion infarction, and it is a short-form measure for assessing the overall neurologic state based on the grasping ability and trunk rotation on a 5-point scale from 0 to 4 points. In both the examinations, the function is considered closer to normalcy when a lower score is achieved.
IHC analysis of brain tissue and the results
Production of brain slices and IHC assessment
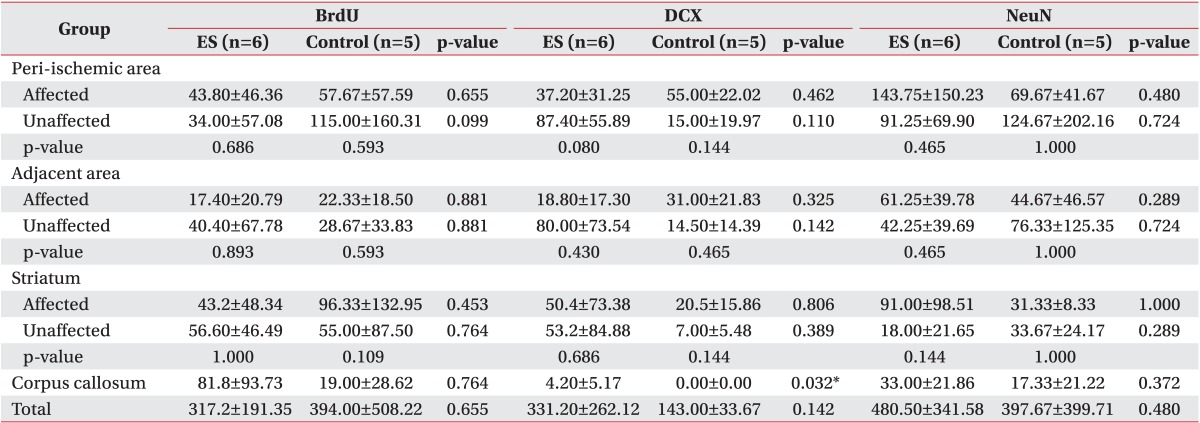
Six rats were randomly selected from each group, and fixation by transcardiac perfusion method after injecting excessive amount of chloral hydrate and post-fixation by dipping the extracted brain into 4% paraformaldehyde for 2 to 4 hours at 4℃ were performed. Fixed brain tissue was sectioned into 4 µm-thick coronal plane slices at every 400 µm by using a tissue cutter RM 2135 (Leica-Microsystems, Wetzlar, Germany) after a series of paraffin penetration treatment. After placing the paraffinized brain slice onto a slide, IHC was performed after de-paraffinization and hydration processes. Three 4 µm-thick interesting coronal brain slices, which were located +1.80 mm within the scope between +2.28 mm and +0.48 mm from the bregma to the frontal region, were selected for IHC. Avidin-biotin-peroxidase complex method was applied for staining, and 3-amino-9-ethylcarbazole (AEC) was also used for color development. Using an optical microscope, the number of positive cells on IHC was counted in each specific region which was configured into one unit at 200× magnifications for statistical analysis. Each specific region was used for an interhemispheric comparison or was compared to the control group by dividing the nearby areas into 7 different sites after establishing the reference point where cerebral ischemia had occurred. These 7 regions comprised of the following: 1, ipsilateral peri-ischemic cortex (IPI, cerebral cortical region including the ischemic penumbra located lateral to the area of cerebral ischemia in the ischemic hemisphere); 2, ipsilateral adjacent peri-ischemic cortex (IAPI, cerebral cortical region located lateral to the region 1); 3, contralateral adjacent peri-ischemic cortex (CAPI, cerebral cortical region in the opposite hemisphere located horizontally from the region 2); 4, contralateral peri-ischemic cortex (CPI, cerebral cortical region in the opposite hemisphere located horizontally from the region 1); 5, ipsilateral striatum (IS, striatum region including the subventricular area in the ischemic hemisphere); 6, contralateral striatum (CS, striatum region including the subventricular area in the opposite hemisphere that was located horizontally from the region 5); and 7, corpus callosum (CC) (Fig. 1).
Seven regions (1-7) in a microscopic field (×200) of an interesting brain slice (+1.80 mm from the bregma, small brain at the bottom) for the measurement of ischemic area and immunostaining. The seven regions were: 1, ipsilateral peri-ischemic cortex; 2, ipsilateral adjacent peri-ischemic cortex; 3, contralateral adjacent peri-ischemic cortex; 4, contralateral peri-ischemic cortex; 5, ipsilateral striatum including the subventricular area; 6, contralateral striatum including the subventricular area; and 7, corpus callosum.
IHC
Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU), neuron-specific nuclear protein (NeuN), and doublecortin (DCX) were used as neuronal markers for assessing the changes in the proliferative function of neocytes/putative endogenous stem cells, mature neural cells, and immature neural cells of neuroblasts, respectively [16,20,21]. For BrdU administration, 100 mg/kg of BrdU (Sigma-Aldrich) was diluted to 25 mg/mL and injected into the abdominal cavity of the subjects at the same time for four days from the second through the fifth day after surgery for inducing cerebral ischemia. After cleansing the brain slice tissue with pH 7.4 Tris-buffered saline plus Tween 20 (ScyTek Laboratories Inc., West Logan, UT, USA) followed by placing it on a UltraVision Hydrogen Peroxide Block (Lab Vision Corporation, Kalamazoo, MI, USA) for 8 minutes at 45℃ to block endogenous peroxidase activity. Brain slice tissues were reacted with primary antibodies such as mouse monoclonal anti-neuronal nuclei NeuN 1:250 (Chemicon International Inc., Temecular, CA, USA), goat polyclonal anti-DCX 1:50 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and rat monoclonal anti-[BU1/75(ICR1)] BrdU 1:200 (Abcam, Cambridge, UK) for 25 minutes at 45℃. Subsequently, the brain slice tissue was allowed to react with secondary antibody (biotinylated anti-mouse IgG) and SuperPicTure Polymer Detection kit (Zymed, San Francisco, USA) combined with HRP polymer for 15 minutes at 45℃, finally followed by color development with AEC (Zymed).
Statistical analysis
A total of 9 cases were excluded; 2 cases from the ES group because of problems with the wires, 2 cases from the control group and 1 case from the ES group because the subjects showed a less than 10% success rate of SPRT at day 1 post-stroke, 1 case from each group wherein the formation of the ischemic lesion was not proper after extraction of the brain, and 1 case from each group wherein the subject deceased on the day after the surgery. Thus, 10 subjects from the ES group and 9 subjects from the control group were eventually studied and analyzed. For performing IHC, 6 subjects from the ES group and 5 subjects from the control group were randomly included for the statistical analysis after excluding 1 case from the control group because it was unsuitable for microscopic examination. For the comparison of behavioral assessment and neurologic examination between the ES group and the control group, independent t-test and Mann-Whitney U test were employed for parametric analysis and nonparametric analysis, respectively; while repeated measures ANOVA was used to investigate the trends of changes between before and after treatment in each group as well as to compare the two groups according to time sequence. Furthermore, Bonferroni test was performed for the correction of multiple comparisons on the 2nd, 5th, 8th, 11th, and 13th day after surgery for induction of cerebral ischemia. After measuring the number of immune positive neuronal cells at 200× magnification, a comparative analysis was conducted between the same regions in both groups as well as between the ischemic hemisphere and the opposite hemisphere within each group. To compare the IHC results between the two groups, Mann-Whitney U test or independent t-test was employed; and Wilcoxon signed rank test was used in order to compare the IHC results between the cerebral ischemic lesion and the corresponding contralesional region. Data was analyzed through SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA) while significance of the statistical value was set at α=0.05, and the difference was considered to be significant when the p-value was less than 0.05. Additionally, the relative ratio of neuronal cells on IHC was calculated in order to understand the trend for an increase in the number of neuronal cells after ES treatment, which was computed by using the equation, [(the number of immune positive neuronal cells in the comparative region - the number of immune positive neuronal cells in the reference region)/the number of immune positive neuronal cells in the reference region]×100% [12]. When the relative ratio was greater than 30% on IHC, it was considered that there was a trend for an increase in the number of neuronal cells [12,22].
RESULTS
Intensity of ES and safety of ES treatment
Movement threshold in this study was from 2.8 to 4.4 V (mean±standard deviation, 3.55±0.67 V). An adverse reaction or an abnormal response to ES such as death, convulsions, behavioral abnormality such as change in consciousness, involuntary movement or neurologic deterioration was not detected during the course of continuous ES of the cerebral cortex that was performed throughout this study [12].
Behavioral assessment after induction of cerebral ischemia
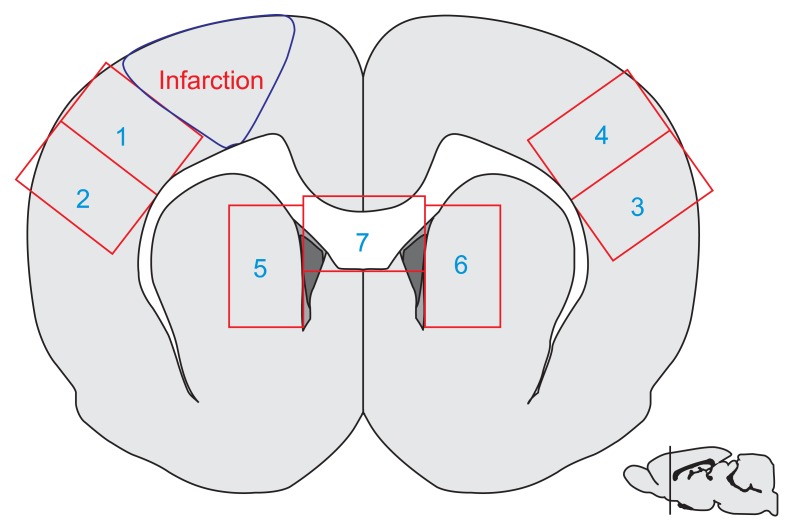
The success rate of SPRT was significantly improved in the ES group compared to the control group on the 5th, 6th, 11th, 12th, and 13th day. The difference in the success rate of SPRT between the two groups was further increased with the passage of time (p<0.05) (Fig. 2). When the success rate of SPRT was compared between the two groups after adjusting for the passage of time on repeated measurements, the success rate of SPRT was significantly improved in the ES group compared to the control group (F=4.504, p=0.047), and the motor function in paralyzed forelimbs within the group was also significantly improved with the passage of time (F=46.869, p<0.001). Most importantly, the success rate of SPRT in the two groups was significantly increased on 5th, 8th, 11th, and 13th day compared to that on the 2nd day after the induction of cerebral ischemia (Bonferroni p<0.05).
Epidural electrical stimulation for 2 weeks after photothrombotic stroke enhanced the motor performance on single pellet reaching task (SPRT). ES, electrical stimulation; Control, the operation control group. *p<0.05 by Mann-Whitney U test between two groups. The comparison of the success rate between groups (p=0.047) and within the group (p<0.001) showed a significant difference in the analysis by repeated measures ANOVA.
Neurologic examination after induction of cerebral ischemia
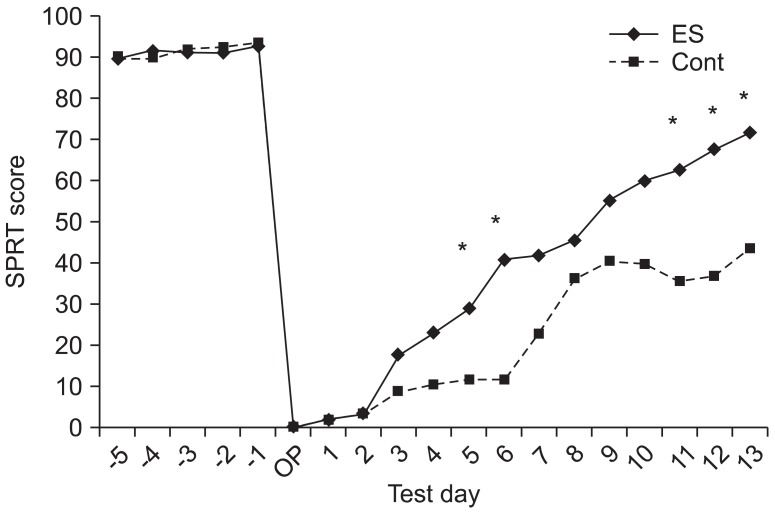
Both the Schabitz test and Menzies test demonstrated a significant recovery from neurologic damage in the two groups with the passage of time after the surgery for induction of cerebral ischemia (Schabitz test: F=51.780, p<0.001; Menzies test: F=19.819, p<0.001) (Fig. 3). More specifically, Schabitz test demonstrated a significantly increased neurologic recovery on the 5th, 8th, 11th, and 13th day compared to that on the 2nd day after induction of cerebral ischemia, whereas Menzies test demonstrated a significantly increased neurologic recovery on the 11th and 13th day compared to that on the 2nd day after induction of cerebral ischemia (Bonferroni p<0.05 in both groups). These two neurologic tests did not show any significant difference between the two groups (Schabitz test: F=0.283, p=0.602; Menzies test: F=0.076, p=0.787) (Fig. 3).
The neurological examinations including Schabitz's photothrombotic neurological score and Menzies test were performed for neurological evaluation of behavioral recovery. There were significant improvements in each neurological test for 2 weeks after photothrombotic stroke (p<0.05), but not between two groups at each test. ES, electrical stimulation; Cont, the operation control group.
IHC of neuronal cells
When the difference in the ischemic hemispheres between the ES group (comparative region) and the control group (reference region) was examined (Table 1), the proportion of NeuN+ cells in the ischemic hemisphere of the ES group was greater by over 30% in IPI (106.33%), IAPI (37.12%), IS (190.46%), and CC (90.42%); the proportion of DCX+ cells in the ischemic hemisphere of the ES group was greater by over 30% in IS (145.85%) and total regions (131.61%), consequently, a significant increase in CC (>420%) of the ES group (p=0.032) (Fig. 4); and the proportion of BrdU+ cells in the ischemic hemisphere of the ES group was greater by over 30% in CC (330.52%).
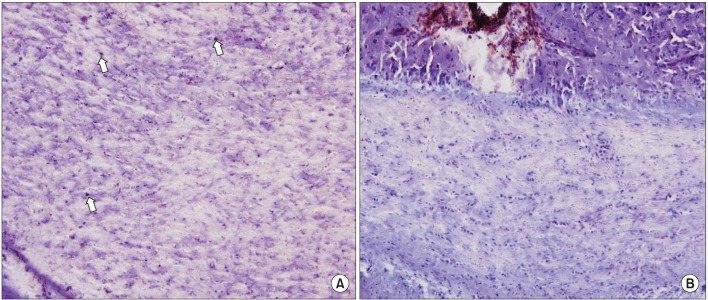
The immunohistochemical findings (×200) of doublecortin staining in the corpus callosum showed a significant increase in the number of neuroblasts (arrows) in the electrical stimulation group (A) than in the control group (B).
When the difference in the ischemic hemispheres between the control group (comparative region) and the ES group (reference region) was examined (Table 1), the proportion of DCX+ cells in the control group was greater by over 30% in IPI (47.85%) and IAPI (64.89%), and the proportion of BrdU+ cells in the control group was greater by over 30% in IPI (31.67%) and IS (122.99%).
When the ischemic hemisphere (comparative region) was compared to the opposite hemisphere (reference region) (Table 1), the proportion of NeuN+ cells in the ES group was greater by over 30% in the ischemic hemisphere-i.e., IPI (57.53%), IAPI (44.97%), and IS (405.56%), and the proportion of DCX+ cells in the control group was greater by over 30% in the ischemic hemisphere-i.e., IPI (266.66%), IAPI (113.79%), and IS (192.86%), while the proportion of BrdU+ cells was greater by over 30% in IS (75.14%).
When the opposite hemisphere (comparative region) was compared to the ischemic hemisphere (reference region) (Table 1), the proportion of DCX+ cells was greater by over 30% in CPI (134.95%) and CAPI (325.53%) of the ES group, the proportion of NeuN+ cells was greater by over 30% in CPI (78.94%) and CAPI (70.88%) of the control group, and the proportion of BrdU+ cells was greater by over 30% in CAPI (132.18%) and CS (31.02%) of the ES group as well as in CPI of the control group (99.41%).
DISCUSSION
It was observed that continuous epidural ES after experimental cerebral ischemia in a rat model of photothrombotic focal ischemia tends to induce a significant improvement in motor function and a positive effect on the neuronal regeneration after cerebral ischemia.
The epidural ES method that was employed in this study did not cause any adverse effect or abnormal response to ES even after it was continuously performed for 2 weeks, which demonstrates that continuous epidural ES treatment is a relatively safe therapeutic option [4,12].
The assessment of SPRT suggested that the motor function was significantly improved compared to that immediately after the surgery in both groups. As time passed, there was a significant recovery of motor function in subjects of the ES group compared to those of the control group, which led to an outcome similar to that in the previous studies. Adkins et al. [16] reported a significant enhancement in motor function for reaching in a rat model of cerebral infarct after application of epidural ES to the ischemic sensorimotor cerebral cortex for 18 days and simultaneous performance of the skilled reaching task after 10 to 14 days post-stroke. As reported above, the application of epidural ES treatment along with skilled reaching task induced a significant improvement in motor function compared to that in the control group in previous studies [3,4,16,23]. However, recovery of motor function and neurologic recovery were also observed when treadmill rehabilitation training was solely performed without application of ES treatment after the induction of cerebral ischemia [20,23]; therefore, there were still some doubts regarding the independent effect of ES. As demonstrated in this study, continuous ES treatment was able to induce a significant improvement in motor function independent of the rehabilitation training. Therefore, continuous epidural ES treatment of the sensorimotor cortex is effective in causing an improvement in motor function, and it is believed to be a safe therapeutic option.
Neurologic examination showed that there was a significant recovery of neurologic function in both groups with the passage of time after the onset of cerebral ischemia. However, time-based neurologic changes did not reveal any significant difference between the two groups. These neurologic examinations are relatively more suitable for evaluating the effect of an intervention such as treadmill training on gross motor function because they mainly show the degree of gross motor function [20,23], the extent of time-based improvement in the individual [20], or these examination criteria are considered to be more useful for determining how a significant lesion was produced after the induction of brain injury such as cerebral infarction [12].
When the IHC differences in neuronal markers within the ischemic hemisphere between the two groups were investigated, it was observed that ES treatment tended to increase the number of mature neural cells in the ischemic hemisphere. A tendency for an increased neural plasticity in the cerebral hemisphere was also demonstrated by proliferation of neuroblasts in addition to mature neural cells in the cerebral subcortical region including the striatum. After observing neuroblasts and neocytes/endogenous stem cells in the corpus callosum of the ES group, it was considered that either the proliferation of these cells was induced in the corpus callosum or the migration of these cells, which proliferated in the opposite hemisphere, to the ischemic hemisphere through the corpus callosum was enhanced compared to that in the control group. Therefore, the outcome reported in the studies by Kleim et al. [10] and Yang et al. [12] that ES improves synaptic reorganization and axonal sprouting in the ischemic penumbra was found to be similar to the outcome of this study.
When the ischemic hemisphere was compared to the opposite hemisphere in order to investigate the effect of ES treatment in the ischemic hemisphere or that of mechanical stimulation such as induction of cerebral ischemia and/or electrode implantation in the ischemic hemisphere, the mechanical stimulation in the control group led to the proliferation of neocytes/endogenous stem cells and neuroblasts, and ES treatment in the ES group for 2 weeks tended to cause an increase in mature neural cells in the ischemic hemisphere. Therefore, it was assumed that ES treatment promptly induced the differentiation of neocytes/endogenous stem cells and neuroblasts into mature neural cells. Jin et al. [21] reported that the number of BrdU- and DCX-incorporated nascent neural cells was increased in the rostral subventricular zone of the lateral ventricle [24], the subgranular zone of the dentate gyrus [25], the ischemic and opposite cerebral hemispheres after the induction of local cerebral ischemia. Thus, this result was similar to that of this study, which demonstrated a tendency for increased neocytes/endogenous stem cells and neuroblasts after the induction of cerebral ischemia. It is already known that the integration or replacement of neocytes to the preexisting neuropil is hard to happen [26,27]; however, increased survival of neocytes under the inadequate environment such as cerebral ischemia is critical for the recovery of motor function. Even in this study, there was a trend for the proliferation of neocytes/endogenous stem cells and neuroblasts in the ischemic hemisphere after the induction of cerebral ischemia, and the increasing number of mature neural cells was evident in the lesion after ES treatment; thus, it was considered that ES treatment in the cerebral cortex can be expected to result in a positive effect in the process of neurogenesis, facilitation of differentiation, and integration of neocytes.
When the opposite hemisphere was compared to the ischemic hemisphere for investigating the effect of the ES treatment in the opposite hemisphere or that of mechanical stimulation such as the induction of cerebral ischemia or electrode implantation in the ischemic hemisphere, the mechanical stimulation tended to increase the number of mature neural cells as well as neocytes/endogenous stem cells in the opposite hemisphere, and if ES treatment was performed in the ischemic hemisphere, there was a tendency for increase in the number of neuroblasts and neocytes/endogenous stem cells in the opposite hemisphere. Therefore, when the ES treatment was applied, it can be considered that the genesis of neocytes/endogenous stem cells and neuroblasts may be increased in the opposite hemisphere since it has a better environment for neural regeneration than the ischemic hemisphere. Afterwards, the possibility of migration of neocytes/endogenous stem cells and neuroblasts from the opposite hemisphere to the ischemic lesion where cerebral ischemia occurred has been suggested. As mentioned previously in the comparison between the ES group and the control group, when considering that the outcome where the number of neocytes/endogenous stem cells and neuroblasts was increased in the corpus callosum, the migration of these cells from the opposite hemisphere to the ischemic hemisphere can be presumed. Aboody et al. [28] reported that the neural stem cells that were implanted in the opposite cerebral hemisphere can migrate towards the tumor region of another cerebral hemisphere, and this suggests the possibility of proliferation of endogenous neural precursor cells in the opposite hemisphere and migration towards the lesion in the ischemic hemisphere; thus, this study by Aboody et al. [28] demonstrated an outcome that was similar to the result of this study. When ES was applied to a unilateral cerebral hemisphere [29], or when traumatic brain injury [30] occurred, neurogenesis was found to be increased in the bilateral subgranular zone or the bilateral subventricular zone; therefore, neurogenesis could be increased in both the cerebral hemispheres.
The limitation of this study was evident when it was difficult to limit the loss of valid subjects due to the characteristics of an animal study. Moreover, since this study focused on the quantitative increase in neural cells using IHC; the fluctuation in the number of positive immune responsive cells and its ratio in each region between subjects was clearly observed. However, this broad distribution was statistically insignificant, and it is believed that the difference may exist in an embryological study, which precisely traced the migration and changes in neocytes/endogenous stem cells as well as neurogenesis and proliferation of neural cells. Therefore, it is firmly believed that more comprehensive and integrated studies are required in the future for assessing the regeneration of neural cells by using a electrophysiological test, a longitudinal follow-up study or a immunofluorescence staining.
In conclusion, through this study, it was confirmed that continuous epidural ES treatment of the sensorimotor cortex in a rat model of photochemically induced cerebral ischemia effectively improved the functional behavioral disorder with motor paralysis. It was also suggested that continuous epidural ES treatment led to histological improvement including the overall proliferation of neuronal cells such as mature neural cells or neuroblasts in the ischemic cerebral hemisphere and increased regeneration of neuronal cells through production of neocytes/endogeneous stem cells and neuroblasts in the opposite hemisphere. Therefore, we suggest that epidural ES provides the environment that is suitable for neural recovery in patients with cerebrovascular lesions and has an advantage over neuroplasty.
Notes
No potential conflict of interest relevant to this article was reported.