Delayed Onset of Thoracic SCIWORA in Adults
Article information
Abstract
Spinal cord injury (SCI) without radiographic abnormality (SCIWORA) is estimated to account for 1-9% of the occurrence of SCI. Of these, cervical SCIWORA in children is common, but thoracic SCIWORA delayed onset in adult is much less common. We experienced a case of 38-years old male patient with lower extremity weakness; he had fallen down a week earlier before the investigation. At the time of admission, motor grade was 4 with voiding incontinence and ambulated with cane. He presented progressive weakness from G4 to G3 and hypoesthesia was below T8 dermatome and ambulated with wheelchair. Whole spine and lumbar MRI findings showed no abnormality and electrodiagnostic findings showed normal NCS, however, abnormal SEP on both the tibial nerves. After steroid therapy and proper rehabilitation program for 2 weeks, lower extremity strength was improved from G4 to G3, voiding was continent, and ambulation reached cane gait.
INTRODUCTION
Spinal cord injury can be caused by diseases such as vertebral displacement or vascular injury due to trauma, inflammation, and tumors. Generally, traumatic spinal cord injury is accompanied by structural defects in the spine or ligament due to external impact. However, as first described by Pang and Wilberger1 in 1982, spinal cord injury without radiographic abnormality (SCIWORA) is a syndrome of spinal cord injury without any evidence of vertebral fracture or bony misalignment as evidenced by plain radiographs or computed tomography (CT). If neurological symptoms are not evident right after the trauma when the radiological findings are not distinguishable, it will be difficult to predict the possibility of spinal cord injury. Until date, the majority of reported SCIWORA cases consist of cervical cord injury in childhood, and injuries besides those in the cervical cord of adults are known to be rare. While the reported latency period of SCIWORA (children) varies from 30 minutes to 4 days, we report a case of an adult male showing thoracic SCIWORA with delayed onset paralysis.
CASE REPORT
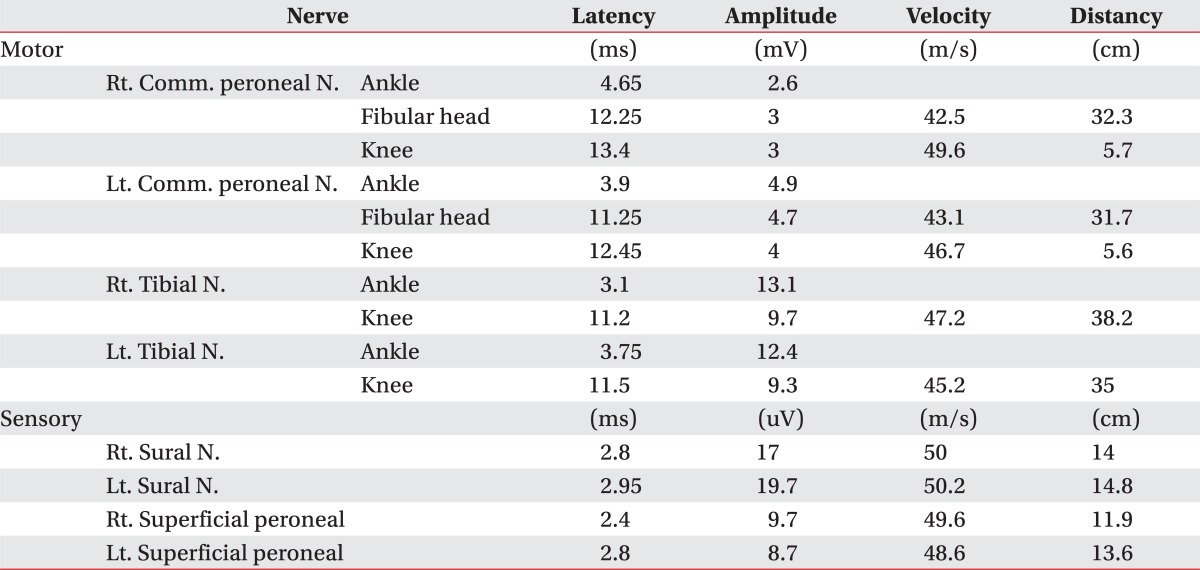
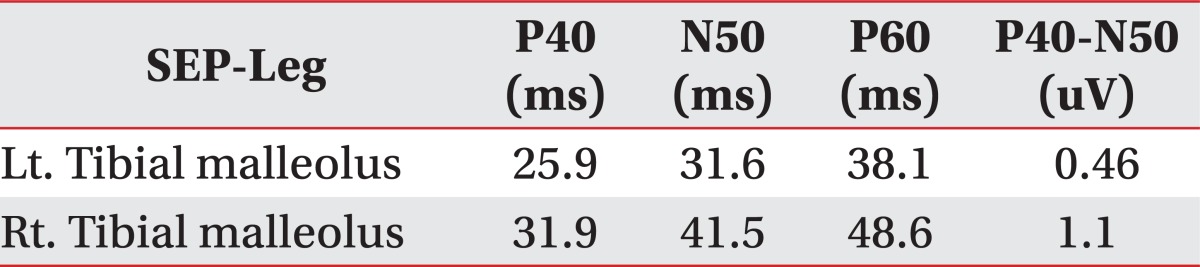
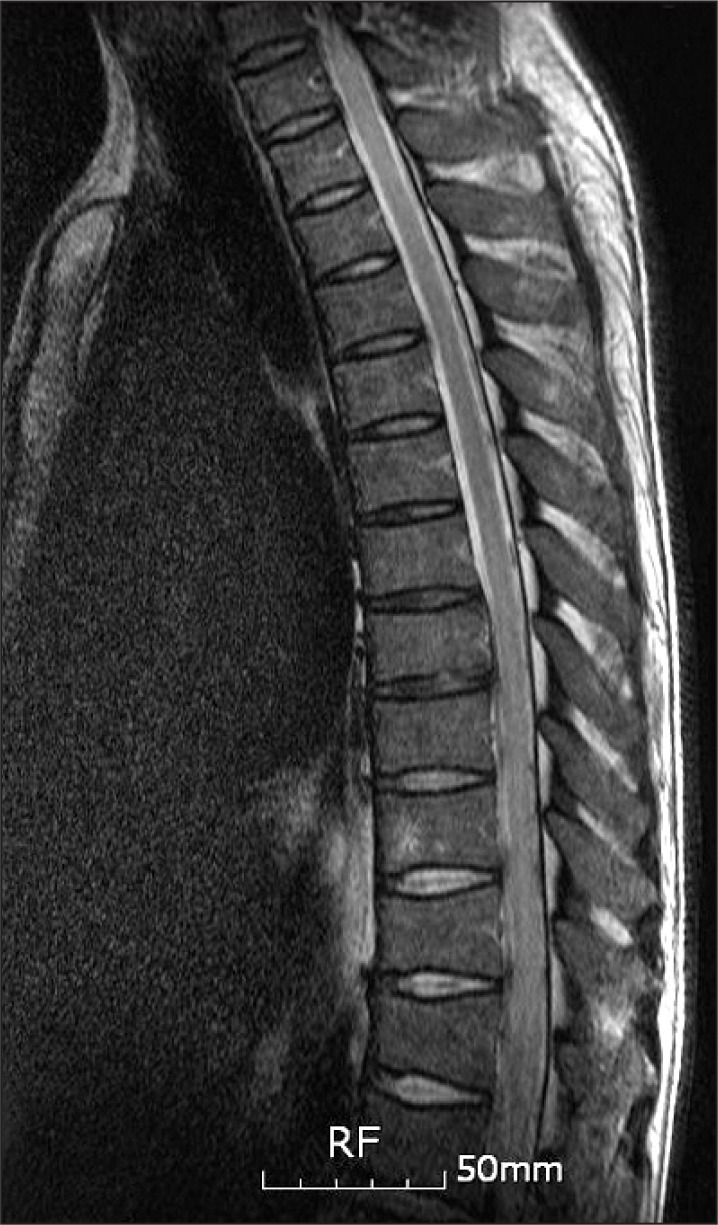
A 38-year-old male had fallen down from a height of about 1.5 m. The patient had no special medical history other than hepatitis B infection and had not experienced back pain before. Since he did not show other neurological symptoms besides back pain at the time he visited a private hospital, he received conservative treatment such as medication and physical therapy. Afterwards, the patient experienced weakness in lower extremity 7 days after the injury while performing his daily routine. At that time, he felt that his trunk was swaying slightly while he was walking. The gait disturbance symptoms gradually progressed and there was onset of additional symptoms such as impotence and lower extremity hypoesthesia. Consequently, he was admitted to the Department of Neurosurgery by the emergency room on the 30th day of the occurrence of injury. No external wound was observed at the time of the patient's hospitalization, and the pain was only in the back and lower extremity. According to the radiologist's reading of the whole spine MRI examination (Fig. 1), there had been findings of a wedge-shaped vertebral body but no findings of spinal cord damage was observed. From the initial blood test, the white blood cell count was 5,900/ul; the segmented neutrophil was 41.3%; the ESR and CRP was within the normal range; and the VDRL, HIV test, plasma lgG, anticardiolipin antibody (ACA), anti-phospholipid, antinuclear antibody (ANA), and blood bacterial culture test results were found to be negative. There were findings of five types of white blood cells, and no lgG in the CSF based on the cerebrospinal fluid test. On physical examination, the bilateral lower extremity strength was of grade 4/5; hypoesthesia of the bilateral extremity; urinary incontinence; and the bowel function was intact. Steroid therapy had been performed under clinical suspicion of cauda equine syndrome. According to the thoracic vertebral MRI, there were no specific findings. On physical examination on the 7th day of the patient's hospitalization, his lower extremity strength deteriorated to grade 3 without any improvement in the symptoms; his sensory functions had been reduced below the T8 dermatome level; Deep Tendon Reflexes were increased; Babinsks's sign was evoked; and urinary incontinence was presented. The neurological level was T8 with ASIA grade D. Functionally, the patient progressed from independent ambulation using canes at the time of his hospitalization to wheelchair ambulation. On the 13th day of hospitalization, as the symptoms worsened, he was transferred to the Department of Neurology, where the second steroid therapy was performed. There were no abnormal findings from the nerve conduction study, H reflex, and F response tests on the lower extremity. The MR brain also showed no abnormal findings (Fig. 2). Since then, the symptoms were maintained without any further worsening. Around the 40th day of the hospitalization (70 days after the injury), he was transferred to Department of Physical Medicine and Rehabilitation. The result of nerve conduction studies were normal and needle electromyography of the L5 paraspinal muscle revealed abnormal spontaneous activity (Table 1). In somatosensory evoked potential obtained by stimulating the tibial nerves, right tibial nerve pathway showed delayed onset latency when compared to left side; while left tibial nerve pathway showed over 50% decrease in amplitude when compared to the right side (Table 2). Afterwards, muscle-strengthening, balance-training, and gait-training using a mono-cane had been performed for one hour each twice a day. According to the follow-up test after two weeks, the deep tendon reflexes of lower extremity and the ankle clonus maintained a positive reaction, and the sensory functions were still in the reduced state. However, the muscle strength improved to grade 4 and the neurogenic bladder was in a condition capable of performing normal bladder functions. Even from the functional aspect, the patient was discharged in a condition in which he can ambulate independently indoors over a distance of 30 m with the help of a cane. Since then, no outpatient follow-up has been recorded.
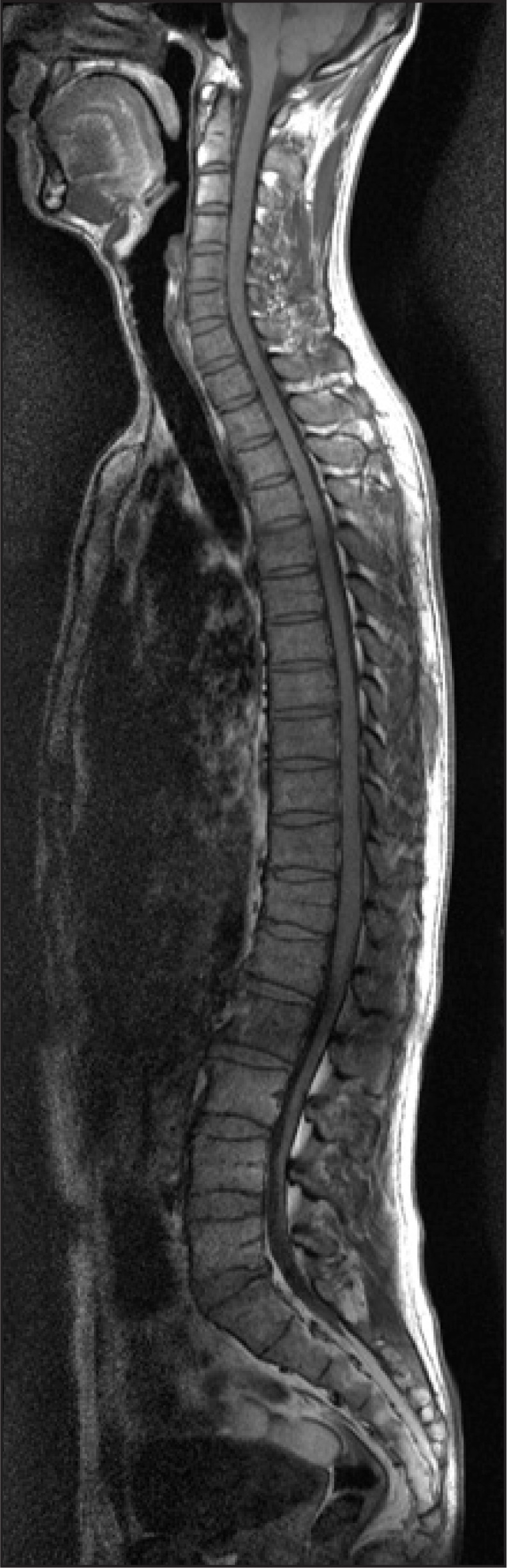
Whole Spine MRI, HD#1. Anterior wedging at T12 and L1, disc degeneration with annular tearing at L1-2.
DISCUSSION
The SCIWORA accounts for approximately 1-9% of all the spinal cord injuries.2 Pang and Pollack2 has classified SCIWORA into four groups based on age. The infant group is generally known to experience the onset of SCIWORA at time of delivery, although the onset is rare. The children's group is known to be prone to SCIWORA due to their natural flexibility and anatomical structure. The adult group (16-45 years old) under which the patient in this case falls, however, is known to have a low incidence rate of SCIWORA, since radiological abnormalities are easily detected,1 although in the report of Anil et al., there are incidences even among young adults with normal vertebral structure.3
While most of the SCIWORA cases are accounted for by cervical cord injuries, the cases by thoracic cord injuries are rare. This is because the splinting effect of the rib cage against the force that arises when the spine bends or extends due to an external impact prevents the horizontal displacement of the spine. However, such an effect of the rib cage is known to be weak against a longitudinal distraction, which describes the possibility of thoracic cord injuries, as in the present case.4
The representative cause of the onset of SCIWORA is explained in the hypothesis of segmental spinal instability, which supports the high rate of incidence of SCIWORA in children. The hypothesis claims that it is relatively easy for a child's spine to return to its normal position after temporal subluxation due to its anatomical features.1 Another hypothesis states that SCIWORA is caused by the differential stretch of the spinal cord and the spine. Besides these, there are vascular and concussive hypotheses, but they lack accurate evidence.5
In the case of this patient, while no other lesion could be found, a focal signal change was suspected that implied intramedullary edema from the thoracic MRI (Fig. 3) taken on the 17th day of the hospitalization. Since it is the thoracic spine where the lesion was relatively stable, it is deemed that the spinal cord injury may have occurred due to the horizontal distraction of the spine due to the temporary hyperextension of the thoracic spine, as seen in the cases of SCIWORA in adults due to minor trauma, as reported by Kim et al.6
MR thoracic, HD#17. No abnormal findings of a thoracic lesion (official reading), but suspicious of a signal change.
Medullary edema after spinal cord injury is generally known to be seen between 24 to 72 hours after the injury. Regarding the timing of the onset of neurological symptoms, however, Hamilton and Myles et al. have stated that the symptoms could occur 4 days after the injury. Ruge et al. reported that paralysis started after a latency period of several days in 52% of children who had SCIWORA, and Osenbach stated that it started in 22% of children with SCIWORA. Frankel et al.7 reported that 2% of adult patients with spinal cord injury had delayed onset of symptoms. While the mechanism of occurrence of medullary edema has not been accurately revealed, three hypotheses have been suggested. The first hypothesis states worsening and progression of the symptoms because of the continuous spinal movement without initial fixation after the occurrence of instability. The second hypothesis deemed it possible when the original cause of the injury is the type that gradually progresses and destroys the nerve tissue, such as in ischemia. The third states it as the gradual progression of edema or hemorrhage.2
In the case of this patient, while the possibility of additional trauma before the hospital visit cannot be completely ruled out, its possibility was low because the patient stated no other traumatic event and there was no external wound. The onsets of neurologic symptoms were delayed until the 7th day of the injury and the symptoms gradually progressed and improved on the 40th day of the hospitalization. It can thus be inferred, as also reported by Kim et al.,6 that there was delayed onset of symptoms due to the deterioration from repeated "punch-drunk" trauma to the cord during the latency period through continuous daily normal activities, instead of immobilization after the occurrence of injury.
As experienced in this case, clinicians should recognize the possibility of SCIWORA caused by the trauma at thoracic level and deterioration after a minor trauma, even among adult patients. Therefore, the precautions must be taken for the proper acute phase management and the preventative treatment. Further studies on the prediction of prognostic factors of delayed onset of SCIWORA are necessary.