Effect of Intradiscal Monopolar Pulsed Radiofrequency on Chronic Discogenic Back Pain Diagnosed by Pressure-Controlled Provocative Discography: A One Year Prospective Study
Article information
Abstract
Objective
To investigate the efficacy and safety of percutaneous intradiscal monopolar pulsed radiofrequency (PRF) in patients with chronic disabling discogenic back pain.
Method
Twenty-six subjects (7 males; mean age 43.2 years) with chronic back pain refractory to active rehabilitative management were recruited. All subjects underwent MRI for evaluation of Modic changes, and monopolar PRF (20 min at 60 V) at the center of target lumbar intervertebral disc confirmed by pressure-controlled provocative discography. Clinical outcomes were measured by the visual analogue scale (VAS), Oswestry disability index (ODI), and sitting tolerance time (ST) for 12 months after treatment. Successful clinical outcome was described as a minimum of 2 point reduction in VAS compared with the baseline at each follow-up period.
Results
The mean VAS for low back pain reduced significantly from 6.4±1.1 at pre-treatment to 4.4±1.9 at 12 months (p<0.05). The mean ODI score was 47.3±15.4 points at pre-treatment and 36.7±19.5 at 12 months (p<0.001). The ST was 27.8±20.4 minutes at pre-treatment and 71.5±42.2 at 12 months (p<0.001). However, successful clinical outcome was achieved at 58%, 50%, and 42%, measured at 3, 6, and 12 months post-treatment. There were no significant relationship between the clinical outcome and Modic changes; no adverse events were recorded.
Conclusion
The results demonstrated that the application of intradiscal monopolar PRF might be relatively effective but limited; successful intervention for chronic refractory discogenic back pain is needed. To achieve the optimal outcome through intradiscal PRF, we suggested further studies about stimulation duration, mode, and intensity of PRF.
INTRODUCTION
Chronic low back pain (LBP) is a common clinical entity problem for the patient and the society. Chronic LBP can be caused by structure-specific etiology including facet joint abnormality, disc pathology, and sacroiliac joint dysfunction, among which discogenic pain had been postulated as an important or common cause.1,2 Clinically, symptoms of discogenic back pain are supposed to be non-radicular pain in the absence of spinal deformity, instability, and neural tension signs.3 The diagnosis can be confirmed by means of provocative discography that can provoke injection-related pain responses, which are area identical or similar to usual LBP symptoms. A control disc, which fails to reproduce pain, should be included.
Vertebral cartilaginous end-plate had been thought to have a crucial role in the mineralization of intervertebral disc.4 Such vertebral end-plate degeneration (Modic change) had been found to correlate with disc degeneration and discogenic back pain.5,6 However, few research had reported such relationship of the presence of Modic changes to the outcome of intradiscal procedures.7,8
Abnormal nerve ingrowth and expression of painful nociceptors had been thought to be the primary etiological factor in discogenic pain.9 Thus, in spite of the difficulty of treating, it appeared promising to modulate ingrowing nociceptors from the outer annulus fibrosus in patients who have discogenic back pain. Many studies investigated the efficacy of the minimally invasive intradiscal procedures, intradiscal electrothermal therapy (IDET), and radiofrequency (RF) ablation for chronic discogenic LBP. This procedure, which was designed to control the intradiscal nerve endings from the outer one third of the annulus fibrosus by thermal energy, had been reported. A portion of LBP patients had improvement in their functional level and achieved symptomatic improvement with these kinds of procedures. However, the effect of minimally invasive intradiscal management has traditionally been limited and is insufficient to satisfy the majority of patients who reported to have discogenic LBP.
In the 1980s, percutaneous RF treatment was introduced.10 RF treatments are divided into continuous radiofrequency (CRF) stimulation and pulsed radiofrequency (PRF) stimulation using an electromagnetic field. CRF treatment uses frictional heat arising from a catheter needle that is designed to deliver RF currents to the surrounding tissues with only its needle tip. Unlike CRF, which delivers a continuous current, PRF delivers high intensity electromagnetic current in pulses, allowing heat to dissipate during the latent period so that neurodestructive temperatures cannot be reached. In several studies, it was reported that PRF stimulation provided pain relief.11-14 Therefore, it has been suggested that the treatment of the intervertebral disc by percutaneous intradiscal PRF might be to reduce nociceptive input from the intervertebral disc. Thus far, two studies15,16 had been performed in order to determine the beneficial effects of intradiscal PRF on discogenic LBP, which showed unexpected promising results compared with other more invasive treatment options for chronic discogenic LBP.17,18 However, replication of similar studies with such evidence-based results should be acquired by other clinical researchers prior to being the standard practice in any clinical practice situation.
Hence, in the current study, we investigated the effects of percutaneous monopolar intradiscal PRF for chronic discogenic LBP subjects, who were earlier diagnosed by pressure-controlled provocative discography.
MATERIALS AND METHODS
Subjects
This single-center clinical trial prospectively evaluated the effectiveness of the PRF procedure in patients with chronic discogenic LBP, which were refractory to the comprehensive conservative medical management, medication, and physical therapy for at least 6 months. Their LBP was greater than their leg pain, commonly exacerbated by sitting, and thus, it was reproduced on provocative discography.
With at least a 1 year of follow-up, all 26 patients were enrolled between April 2008 and April 2010 and met the specific eligibility criteria provided in Table 1.
Analysis of MRI
The lumbar spine MRI was performed to grade the end-plate marrow changes of the target discs. We described type 1 as hypointensity on T1-weighted images and hyperintensity on T2, type 2 as hyperintensity on T1 and T2-weighted images, and type 3 as hypointensity on T1 and T2-weighted images.6
Interventions
Patients were informed about the procedure and the way discogenic pain could be provoked by discography. The discography was applied to the affected disc level(s) with one control disc. Briefly, using an oblique projection and a tunnel vision technique, a 22-gauge, 90 mm needle was introduced just anterior to the superior articular process and parallel with the fluoroscopic beam. After the needle tip was in contact with the outer annular fibrosis of the target disc, a 25-gauge, 120 mm needle was inserted in it and was aimed at the center of the affected disc. The needle tip was finally secured in the exact center of the intervertebral space on the antero-posterior and lateral views. The needle tip was then connected to the automated pressure-controlled discography APCD (Cybermedic Corp., Iksan, Korea).19 Injection speed of contrast was set to 0.02 cc/sec in all patients. Then, intradiscal pressure and injected volume were measured in real time. If the intradiscal pressure exceeded 50 psi above the static opening pressure, or the volume exceeded 3.5 cc, or VAS score exceeded 6 point, the discography was terminated. Discography was performed for all patients at L3-4, L4-5, and L5-S1 levels in order to verify the particular disc levels in which concordant pain was provoked. In addition, L2-3 disc was also examined with the spinal puncture in some patients who had reported such provoked concordant pain at all three disc levels. This is because we do not have any control disc when all three disc levels were afflicted with concordant pain. Physical therapy and rehabilitation after the PRF procedure were initiated approximately 4 to 6 weeks post-procedure, and were administered by independent therapists not associated with the investigator.
Intradiscal PRF was conducted within 7 days after discography. The level(s) concordantly reproducing the patients' pain in discography was (were) selected for the PRF treatment. A 20-gauge SMK C15 cannula with a 15-mm active tip (Cotop International BV, Amsterdam, The Netherlands) was placed at the center of the affected disc using antero-posterior and lateral views. The parameters applied for PRF using an RF generator RFG-1A (COSMAN Medical Inc., Burlington, USA) were as follows: frequency 2, 20 milliseconds pulse width, and 60 V for 20 minutes.
Outcome measurements
All patients were subjected to an extensive questionnaire that included the visual analogue scale (VAS: 0-10), sitting tolerance time (ST) without LBP, and Oswestry disability index (ODI) before commencing any form of treatment. VAS was used to measure the intensity of the subjects' pain complaint. ODI and ST were used to evaluate the functions affected by this pain. Patient outcome measures were evaluated before treatment and also at 3, 6, and 12 months after the PRF procedure. Successful clinical outcome was described as moderate when there was over a 2 point reduction in VAS to below 50% pain reduction, and good when 50% or more pain reduction was reported.
Statistical methods
The VAS pain scores, ST, and ODI scores were tabulated. Pre- and post-treatment ranges, means, and standard deviations (SD) were ascertained. Statistical analysis was performed using SPSS 14.0 for Windows, and the clinical course of VAS, ODI, and ST was analyzed via Friedman test, which is a non-parametric repeated measures comparison. Bonferroni correction was applied to avoid statistical error induced by multiplication of the tests (p<0.005). Linear regression analysis was performed for ODI, VAS, and ST, as well as for Modic change. A statistical significance was obtained at a p-value of less than 0.05.
RESULTS
Twenty-six patients (20 women; mean age 43.2±11.8 years) were included in this study. The mean duration of LBP at the entry to the study was 26.8±19.7 months. Twelve patients received intradiscal PRF at 1 spinal level; another 12 had intradiscal PRF at 2 spinal levels, and two patients at 3 spinal levels. Consequently, the number of levels at which PRF had been stimulated was 42 discs. Modic I changes were seen in 2 patients (8%), Modic II changes in 9 patients (35%), and Modic III changes in no patients. In 15 patients (58%), there were no Modic changes evident; however, it only showed positive discography with disc degeneration. However, there was no definite correlation between treatment outcome and presence of Modic changes at the linear regression analysis.
The mean VAS of LBP was 6.4±1.1 prior to the intradiscal PRF treatment, 4.1±1.8 at 1 month, 4.1±1.9 at 3 months, 4.2±2.0 at 6 months, and 4.4±1.9 at 12 months (p<0.05) (Fig. 1). After 3 months, clinical success was achieved in fifteen (58%) of the 26 patients. Only ten (38%) patients reported good clinical success, which was assessed as being over 50% improvement in VAS. However, eleven patients (42%) experienced no clinical improvement of back pain after intradiscal PRF procedure. After 12 months, only eleven (42%) still remained in the clinical success group, yet, fifteen (58%) experienced no clinical improvement (Table 2).
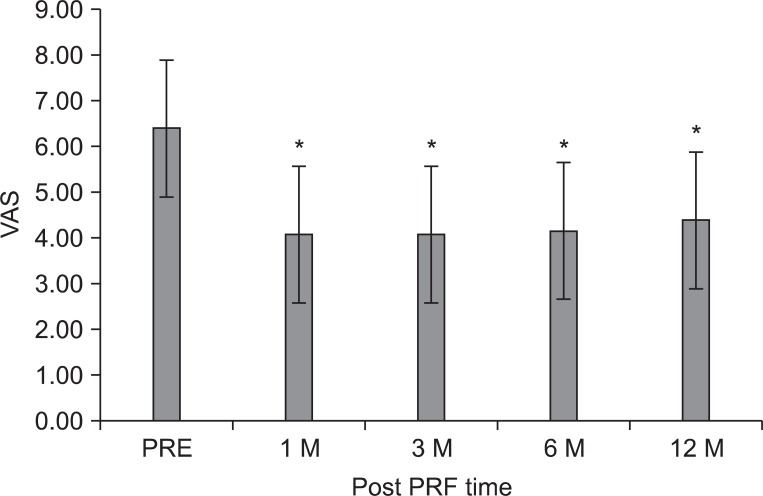
The mean visual analogue scale (VAS) of patients for lower back pain was 6.4±1.1, prior to the intradiscal pulsed radiofrequency (PRF) treatment, 4.1±1.8 at 1 month, 4.1±1.9 at 3 months, 4.2±2.0 at 6 months, and 4.4±1.9 at 12 months after treatment. The magnitude of change was relatively small. The data were expressed as the mean VAS ± standard error. PRE: Pretreatment of PRF. *p<0.01 compared to the pretreatment of PRF.
The mean ODI score before treatment was 47.3±15.4 points, subsequently 32.4±18.5, 34.0±18.1, 34.0±18.1, and 36.7±19.5 points at 1, 3, 6, and 12 months (p<0.001) (Fig. 2). The ST was 27.8±20.4 minutes at pretreatment, 70.8±43.2 at 1 month, and 78.5±42.2 at 3 months, 71.5±42.2 at 6 months, and 71.5±42.2 at 12 months, which corresponded to a mean improvement of 137, 171, 156, and 156%, respectively (p<0.001) (Fig. 3).
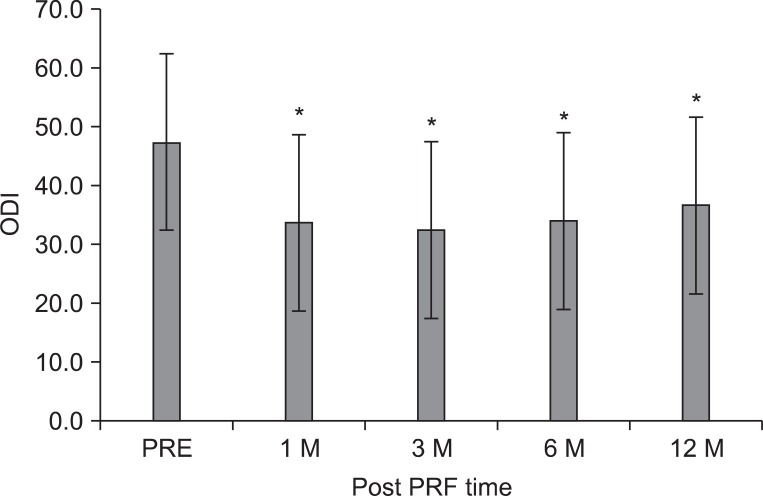
The mean Oswestry disability index (ODI) score was 47.3±15.4 points before the intradiscal pulsed radiofrequency (PRF) treatment, 33.7±18.3, 32.4±18.5, 34.0±18.1, and 36.7±19.5 points at 1, 3, 6, and 12 months after treatment, respectively. The magnitude of change was relatively small. The data were expressed as the mean ODI±standard error. PRE: Pretreatment of PRF. *p<0.05 compared to the pretreatment of intradiscal PRF.
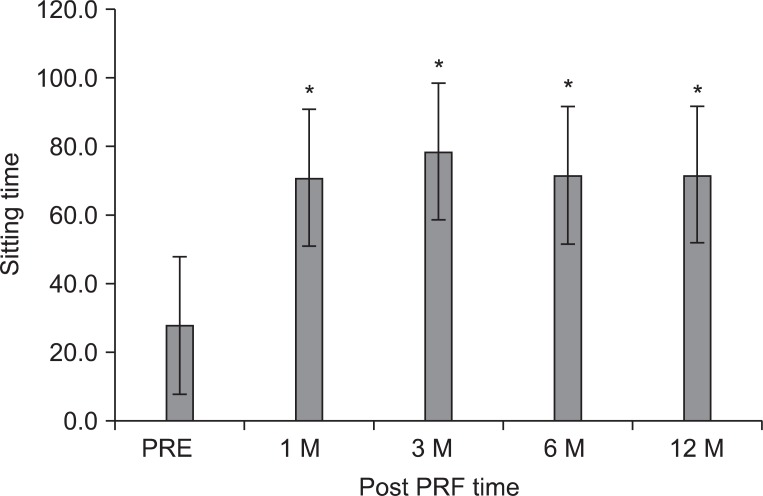
The mean sitting tolerance time (ST) was 27.8±20.4 minutes at pretreatment of the intradiscal pulsed radiofrequency (PRF), 70.8±43.2 at 1 month, 78.5±42.2 at 3 months, 71.5±42.2 at 6 months, and 71.5±42.2 at 12 months after treatment, respectively. The data were expressed as the mean ST±standard error. PRE: Pretreatment of PRF. *p<0.05 compared to the pretreatment of intradiscal PRF.
In all 26 patients treated with intradiscal PRF, no complications occurred during procedure and no post procedural adverse events, such as infections or neurologic sequelae, were reported.
DISCUSSION
This research performed PRF treatment on patients who were diagnosed with pain provocative discography, and who complained of continued chronic discogenic back pain for a minimum of 6 months even after conservative treatment (medication and physical therapy). In the present study, patients treated with intradiscal PRF achieved only a 42% clinical success rate in VAS score of LBP at 12 months after treatment. VAS, ODI, and ST on discogenic pain decreased significantly at 1 month, and maintained a reduction of pain intensity and functional improvement at 12 months after treatment, even though the magnitude of change was relatively small.
Human beings' nerves, containing putative nociceptive neurotransmitters, extend no further into the intervertebral disc than the outer third of the annulus fibrosis. However, degenerated disc producing discogenic back pain had nerves reportedly to be present in the inner third of the annulus fibrosis.20,21 Therefore, attempts have been made to denervate the nerves of degenerative disc with heat lesion produced by several different procedures. Many studies investigated the efficacy of the minimally invasive intradiscal procedures, IDET, and RF ablation for chronic discogenic LBP. First, IDET applications of thermal energy to the painful disc induce collagen fibril denaturation in the posterior annulus fibrosus and destroy nociceptors in the annulus.22-24 Wetzel et al.23 reported the effect of IDET in 75 patients with degenerative lumbar disc diseases. The pain intensity reduced the mean by 2.4 (ranged from 6.0 to 3.6) at the time of the 12 month follow up, and failure rate was 14.7%. However, Pauza et al.22 reported that approximately 50% of the patients experienced no appreciable benefit in a randomized, placebo-controlled trial of IDET. Therefore, the IDET is not clear in its efficacy on discogenic LBP and neural deafferentation, or with collagen modulation. Second, RF is the method using the spread of heat into the nucleus pulposus, and as a result, improvement of pain or disability has been reported. In fact, the CRF procedures that were previously utilized to treat lumbar facet joint pain demonstrated effectiveness through the application of heat and subsequent destruction of afferent nociceptive fibers.25 Thus, it has been suggested that percutaneous CRF might be used to reduce nociceptive input from the intervertebral disc, and several studies reported the effectiveness of intradiscal CRF therapy.26 However, Gerard et al. reported that percutaneous intradiscal CRF (90 seconds, 70℃) is not effective in reducing chronic discogenic LBP. A recent study by Houpt et al. demonstrated that, using the 90-second 70℃ CRF therapy of the intervertebral disc in an experimental situation, temperature changes at distances further than 11 mm from the thermistor tip were insufficient to increase the temperature above 42℃ needed for neuronal cell death.27 Hence, thermal neurodestructive method of CRF was apparently not sufficient to manage discogenic pain.
Recently, neuromodulation of PRF by an electromagnetic field treatment was introduced.28 Exposure of PRF to the dorsal root ganglion can affect cellular function in the dorsal horn of the spinal cord, independently of thermal effects.29 Apparently, such electromagnetic field of PRF may enhance descending inhibitory pathways, specifically involving the noradrenergic and serotonergic systems.30 In addition, the nerve damage appears to be more pronounced for C-fibers, known as principal sensory nociceptors, than for A-δ and A-β fibers.31 Therefore, we assumed that a PRF treatment in the nucleus would change the conductivity of nerve endings that have been sprouting into the degenerated nucleus and modulate pain sensitivity. However, the electromagnetic field was focused at the center of the target disc rather than on the outer one third, more sensitive and essential area, in order to produce discogenic pain. For this reason, the central localization of the needle tip may have a negative effect on the efficacy of intradiscal monopolar PRF. In view of the improvement of outcome through localization of the needle tip, a navigatable PRF device needs to be developed in the future.
To our best knowledge, to date, there were two reports about the effect of intradiscal monopolar PRF. Teixeira et al. reported the effect of intradiscal PRF (for 20 min at 60 V with a 15-min active tip) treatment in eight patients via a numeric rating scale, and all patients had a fall of at least 4 points at the 3-month follow-up.15 Another study by Rohof reported that 38% of the patients had >50% pain reduction at 3-month; at 12 month, the effect was maintained in 29, and of all patients, 56% had >50% pain reduction 1 year after treatment. The parameters applied for PRF were 60 V for 15 minutes with a 2-cm active tip.16 These researchers revealed the unexpected promising efficacy of intradiscal PRF for discogenic pain compared to their other minimal invasive techniques, because intradiscal PRF was more simple and less time-consuming compared to previous intradiscal RF procedures. The clinical outcome of our study was apparently worse than that of previous studies. Although we postulated our difficulty to evaluate the cause of such reported differences from that of other studies, it might have been attributed to the differences in the method of discography. Contrary to previous studies using manually controlled discography, our study utilized automated pressure-controlled discography, which reflected real-time changes in the pressure with contrast material injection. This apparently led to the improvement in the quality of data and the reliability of our discography method.32 Contrary to the possibility of over diagnosis of discogenic pain via manual discography reportedly used in previous studies, there might be a possibility of underdiagnosed discogenic pain among untreated discs in our study due to a small 25-gauge needle, slow injection speed (0.02 cc/sec), and 50 psi as cut-off pressure.33
Our study evaluated the efficacy of intradiscal PRF from functional outcomes, sitting intolerance, and ODI. In particular, sitting intolerance by discogenic pain in Korea interferes with the activity of daily living and social activity because of the cultural preferences for floor sitting during most activities of daily living in Asian countries. In the present study, we demonstrated that sitting time without LBP increased from about 27.8 minutes at pretreatment to 71.5 minutes at 12 months after treatment. This increased sitting tolerance on the floor in preference to a chair-sitting might help subjects manage to live without much difficulty in our society.
End-plate degeneration had been regarded as a factor to have a correlation with discogenic pain.5,34 Modic changes, which involved disc and end-plate degeneration, are in some cases the result of mechanical and nutritional causes.35 Histologic specimen showed fibrovascular replacement in Modic type I degenerative disc.6 These changes give rise to the ingrowing nerve into the inner third of the annulus fibrosus and inflammation. So, Peng et al. reported that intradiscal steroid injection was effective for patients with positive discography and end plate Modic changes in a randomized controlled study.36 Therefore, we assumed that there should be a relationship with the outcome of intradiscal PRF and Modic changes. However, there was no definite statistical evidence in our data. Zhuang's study provides that there is no significant difference in the efficacy of intradiscal steroid injection between Modic types.37 Hence, the efficacy of intradiscal procedures according to the type of Modic change is not fully known. Although this study has a limitation of a small number of investigated patients, no significant correlation between efficacy of intradiscal PRF and Modic changes was found. This implicates that the effect of intradiscal PRF for discogenic pain might not be so much related to anti-inflammation.
In the mechanism of intradiscal PRF, it is assumed that a PRF stimulation in the nucleus would change the conductivity of nerve endings that have been sprouting into the nucleus.16 Nevertheless, the mechanism of the beneficial effect of intradiscal PRF has not been fully elucidated. A change in conductivity of sprouted nerve ending might probably show a much better ST outcome as compared to the other parameters measured, resulting in poorer outcomes other than ST. In our professional assessment, floor-sitting situations would be much worst in causing such discogenic LBP relative to chair-sitting in the Western culture. Yet, our data supported the PRF application to result in a much better ST for floor-sitting, which might be attributed to these change in nerve conductivity due to PRF procedures.
Pauza et al. reported that a decrease in VAS 6 months after treatment in the IDET group and sham group was 2.4 and 1.1, respectively.38 Our outcome, 12 months after intradiscal PRF, seems be slightly inferior to the outcome of the IDET group. However, the advantage of monopolar intradiscal PRF procedure is simple and less invasive compared to the other intradiscal procedure, and there is less possibility of making disc degeneration due to the procedure itself. So, we postulate that further evaluation of mechanism linking intradiscal PRF to improve discogenic pain is needed using an animal model. This study also reported that intradiscal PRF treatment appeared to be relatively safe without neurologic deficits because serious adverse events were not observed over the course of the study, even though we cannot state this definitively due to the small study population. In the future, large cohort studies should be conducted in order to establish the safety issue.
The limitations of this study were that it was not controlled, and in addition, the number of patients was not sufficient. We cannot exclude the possibility of physiologic healing over time. However, to lessen the possibility of natural improvement without PRF treatment in this study, subjects who had shown no interval change of their pain intensity despite conservative treatment for at least 6 months were chosen. To achieve the optimal outcome through intradiscal PRF, further study should be needed about stimulation duration, mode, and intensity of PRF, which is yet to be established. In addition, a follow-up of MRI should be conducted in order to determine the potential to prevent or to aggravate disc degeneration by intradiscal PRF.
CONCLUSION
We concluded that the application of intradiscal PRF might be relatively effective, however, successful intervention for chronic refractory discogenic back pain may be limited. To achieve the optimal outcome through intradiscal PRF, further study is required as to stimulation duration, mode, and intensity of PRF, as well as to explore the action mechanisms to reduce discogenic pain.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Health care technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084177).