Neuromuscular Electrical Stimulation Therapy for Dysphagia Caused by Wilson's Disease
Article information
Abstract
Wilson's disease is an autosomal recessive disorder of abnormal copper metabolism. Although dysphagia is a common complaint of patients with Wilson's disease and pneumonia is an important cause of death in these patients, management of swallowing function has rarely been reported in the context of Wilson's disease. Hence, we report a case of Wilson's disease presenting with dysphagia. A 33-year-old man visited our hospital with a complaint of difficulty in swallowing, since about last 7 years and which had worsened since the last 2-3 months. He was diagnosed with Wilson's disease about 13 years ago. On the initial VFSS, reduced hyoid bone movement, impaired epiglottic movement and moderate amount of residue in the valleculae during the pharyngeal phase were noted. After 10 sessions of neuromuscular electrical stimulation for 1 hour per day, decreased amount of residue was observed in the valleculae during the pharyngeal phase on the follow-up VFSS.
INTRODUCTION
Wilson's disease is a rare, autosomal recessive genetic disorder in which copper accumulates in multiple organs especially the liver, brain, kidneys and the cornea. This is caused due to a drainage disorder of copper through the biliary tract as a result of damage to the 13th chromosome.1 Wilson's disease occurs at the rate of 1 per 30000 people and causes neurological symptoms owing to the deposition of copper in the basal ganglia. Some of the symptoms include: motor disturbance, spastic muscle tension disorder and Kayser-Fleischer ring. It is known that 85% of local patients of Wilson's disease have liver disease symptoms and 35% have neurological symptoms. Among them, neurological symptoms mostly occur in adolescents above the age of 15 years and they present with tremors, limb rigidity, dysphonia and hypersalivation. Dysphagia is one of the neurological symptoms, occuring in more than 50% of Wilson's disease patients. This symptom worsens as the disease progresses and finally enteral feeding is required. If enteral feeding is not provided, there is a large possibility of occurrence of aspiration pneumonia that debases the patient's quality of life.1,2 The effect of neuromuscular electrical stimulation as a treatment for dysphagia caused by Wilson's disease has never been reported in South Korea. In this study, we examined the swallowing function in the dysphagic patient diagnosed with Wilson's disease and have reported our experience that neuromuscular electrical stimulation improved the dysphagia in this case.
CASE REPORT
A 33-year-old man had difficulty in swallowing solids and liquids since the last 7 years. This difficulty in swallowing was aggravated since the last 2-3 months prior to hospitalization. His medical history revealed that he was diagnosed with Wilson's disease in 1997 and he had no other diseases. His family history revealed that his elder sister died of Wilson's disease 13 years ago. The physical examination showed that the cognitive function was normal while dysarthria was evident. The evaluation of cerebellar function tests showed mild ataxia and dysdiadochokinesia. The limb muscle strength testing using the MRC (Medical Research Council) demonstrated normal results within a range of 5 degrees and no muscle atrophy. Generalized distal sensory disturbance was observed in both the upper and lower limbs, and normal deep tendon reflexes and a normal gag reflex were also observed. The laboratory tests showed that the amount of serum copper and ceruloplasmin level was reduced; 21.15 ug/dl and 8.3 mg/dl respectively. The abdominal ultrasound showed liver cirrhosis with significant splenomegaly. As far as the radiologic finding is concerned, the brain magnetic resonance imaging (MRI) on FLAIR (Fluid attenuated inversion recovery) imaging showed high signal intensity in both the pons and the midbrain (Fig. 1).

Brain magnetic resonance imaging on fluid attenuated inversion recovery imaging shows high signal intensity (arrow) in the pons (A) and the midbrain (B).
Videofluoroscopic swallowing study (VFSS) findings were evaluated according to the functional dysphagia scale.3 The patient was placed on a chair in an upright sitting position, and a lateral view was taken and then the food was swallowed in regular order. The food bolus was kept on the fluoroscopic screening field throughout the swallowing process. For the test diet, we sequentially used: 1) 2 ml of 35% w/v liquid barium dilution; 2) 5 ml of 35% w/v liquid barium dilution; 3) 2 ml of barium-blended yogurt; 4) 5 ml of barium-blended yogurt; 5) barium-blended mashed banana; 6) barium-blended soft diet; and 7) barium-blended cookies. The result of VFSS was divided into three phases: the oral preparatory phase, the oral phase, the pharyngeal phase and each phase was checked for abnormalities. Images of the VFSS were recorded at 30 frames per second and analyzed by one of the authors using a multimedia player. A circular metal 2-cm in diameter was attached to the cervical spine of the patient, and the ascent rate of hyolaryngeal complex was compared with the diameter of the metal on five continuous occasions.
As for the results of the VFSS, no significant findings in mastication and lip closure were seen in the oral preparatory phase, but tongue thrust, prolonged oral transit time (>1.5 sec.) and a small amount of residue (<10%) in the oral cavity were observed in the oral phase. The pharyngeal phase showed reduced movement of the hyolaryngeal complex of 1.48 cm, whereas this is about 2 cm in a normal person.4 Also, slow progress of food including diluted barium, yogurt, bananas, soft diet, cookies was observed, and moderate (10-50%) amount of residue in the valleculae was also observed. However, no laryngeal penetration or aspiration was noted.
After the VFSS, both the oral and pharyngeal phase abnormalities were detected, but the patient was treated with neuromuscular electrical stimulation therapy NMES, VitalStim® (Chattanooga Group, Austin, USA) only due to the patient's refusal of other traditional dysphagia treatment.
NMES was performed for 1 hour per day, 5 times a week for 2 weeks; a total of 10 sessions. After positioning the electrical pads, channel 1 was attached horizontally just above the hyoid bone, channel 2 was attached between the thyroid cartilage and the annular cartilage and an additional channel 2 was attached vertically under the annular cartilage. With this placement, the current of the channel 1 was focused on the mylohyoid, digastric and thyrohyoid muscles, and channel 2 sent current through the infrahyoid muscles and intrinsic laryngeal muscles. The current-sending channel 2 stimulates laryngeal elevation. The intensity of the stimulus was increased gradually in 0.5 mA increments from 0 mA until spontaneous swallowing occurred without severe pain, and we encouraged the patient to determine the treatment intensity at a level of more than 7 mA. The patient ingested yogurt during the 1-hour treatment in order to provide an electrical stimulus and voluntary stimulus to the pharyngeal muscles at the same time. After 10 sessions of NMES, we compared the results of the post-treatment VFSS with those of the pre-treatment VFSS. No significant difference was seen between the post-treatment VFSS findings and the pre-treatment VFSS findings in the oral phase, and a movement of the hyolaryngeal complex of 1.52 cm was noted. But in the pharyngeal phase, the amount of vallecular residue was reduced (<10%) in the post-treatment VFSS than that in the pre-treatment VFSS (Fig. 2-C, D).
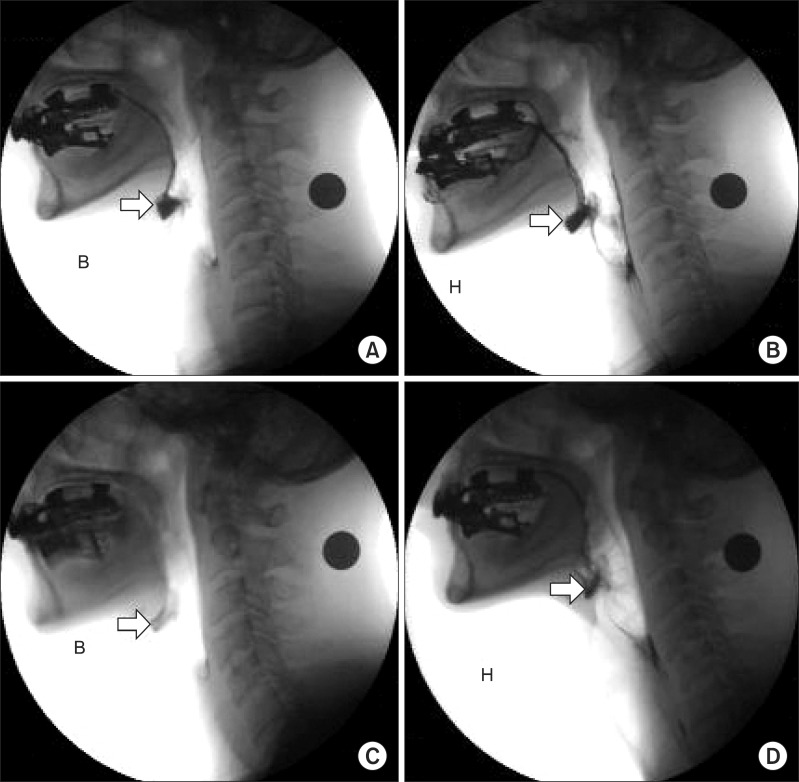
Videofluoroscopic swallowing study images were taken after swallowing bananas (A, C) and cookies (B, D). (A, B) the initial video fluoroscopic swallowing study demonstrates a moderate amount of residue in the valleculae (arrow). After 10 sessions of neuro muscular electrical stimulation, the follow-up VFSS was performed. (C, D) each examination shows a lesser amount of residue in the valleculae (arrow) compared with that in the previous test (B: banana, H: cookies).
DISCUSSION
Dysphagia occurs in 50% of patients of Wilson's disease and it debases the patient's quality of life terribly in the late stage of the disease. da Silva-Júnior et al.5 reported that pre- and post-synaptic dopamine deficiency due to accumulation of copper in the brain, especially in the substantia nigra causes dysphagia, hence dysphagia in Wilson's disease could show a similar pattern to dysphagia in Parkinson's disease. Hence, they performed scintigraphic examination and described the characteristics of dysphagia caused by Wilson's disease as prolonged oral transit time, greater percentage of oral and pharyngeal residue, and slower pharyngeal transit time when compared to age-matched healthy individuals.
Regarding dysphagia in Parkinson's disease, Pfeiffer6 reported that all stages of swallowing - the oral, pharyngeal, and esophageal stages could be affected. Rigidity, bradykinesia, and even tremor of the tongue and oral musculature may impede bolus formation and slow oral transit time. Also, pharyngeal dysmotility may result in misdirected swallows, which increase the risk of aspiration.
Gulyas and Salazar-Grueso2 have reported the VFSS findings in appro ximately 49-year-old female patients with Wilson disease. They found that dysphagia in Wilson's disease shows residues in the valleculae and pyriform sinuses with pharyngeal and esophageal dysmotility. These results were similar to those in our case study since decreased laryngeal elevation and moderate amount of residue in the valleculae were seen in the pharyngeal phase of our study as well.
Logemann7 have reported that the effects of VitalStim in the treatment of dysphagia. He suggested that much more research will be needed to determine if VitalStim plays any role in the management of oropharyngeal swal lowing difficulties in any specific group of patients. On the other hand, Langdon and Blacker8 discussed regarding NMES as a treatment of dysphagia after stroke. They reported NMES as a newer adjunctive treatment modality for dysphagia in stroke. They also described that there is a good theoretical basis to support the use of NMES as an adjunctive therapy in dysphagia and there would appear to be a great need for further well-designed studies to accurately determine the safety and efficacy of this technique. Also, Shaw et al.9 have reported their study results stating that NMES was used in 18 dysphagic patients and it improved the dysphagia in 11 of 18 patients (61%).
But no reference about the effectiveness of NMES for dysphagia in Wilson's disease has been found till date.
Crary et al.10 described two surveys and reported the VitalStim therapy protocol. Most respondents treated patients on an hourly basis on either a three-day-per-week (39% vs. 40%) or a five-day-per-week (42% vs. 38%) schedule. Patients most frequently received a total of 11 to 15 treatment sessions (38% for both groups) or 16 to 20 treatment sessions (25% vs. 16% respectively).
In this case, the patient underwent NMES for 1 hour per day, for a total of 10 sessions, and the amount of vallecular residue was reduced after the treatment. We hereby report our experience that the dysphagia in the Wilson's disease patient improved with the NMES.