Cefepime Neurotoxicity in Patients with Renal Insufficiency
Article information
Abstract
Cefepime is a fourth-generation cephalosporin that is active against both gram-positive and gram-negative organisms. It is administered parenterally for the treatment of severe infections. Approximately 85% of the drug is excreted unchanged by the kidneys. Neurotoxicity in patients with renal failure who are treated with cefepime has been reported sporadically. We report on two senile patients with renal impairment who developed neurotoxicity including lethal outcome after treatment with cefepime.
INTRODUCTION
Cefepime, is a β-lactam antibiotic and a fourth-generation cephalosporin that is highly effective against both gram-positive and gram-negative bacteria. It is widely parenterally administered for severe infections.1 Eighty-five percent of the drug is excreted via the kidney, and adverse events in the central nervous system (CNS) related to encephalopathy have been sporadically reported in patients with decreased renal function.2,3 Recently, cases of neurotoxicity were reported in patients with normal renal function,4 although the mechanism of the neurotoxicity was not established. Such cases of CNS toxicity are rare worldwide. We experienced two cases of neurotoxic adverse events related to cefepime that occurred in two elderly patients. These cases are reported here.
CASE REPORTS
Case 1
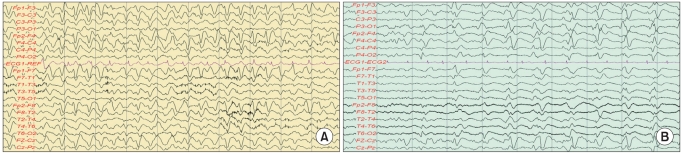
A 74-year-old male patient who had been undergoing hemodialysis for end-stage renal insufficiency for the previous 2 years underwent a laminectomy and L5 irrigation at the Department of Orthopedics of Konkuk University Medical Center due to epidural abscess and septic spondylitis at L4-5. After the surgery, the patient experienced gait disturbance due to cauda equina syndrome and was transferred to the authors' department for rehabilitation therapy. After surgical treatment for septic spondylitis, 1 g/day of cefepime was intravenously administered for 3 weeks. Blood chemistry findings such as acute inflammatory reaction levels stabilized, and the drug treatment was changed to a combination of amoxicillin and ciprofloxacin. The patient was on this combination drug for about 2 weeks. Febrile findings emerged, however, and the patient's blood chemistry revealed increases in erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level, which reflect acute inflammatory levels, to 53 mm/h (normal range 0-15 mm/h) and 6.27 mg/dl (normal range 0.01-0.3), respectively. Also, blood urea nitrogen (BUN) and creatinine, which reflect the level of renal function, had increased to 31.2 mg/dl (normal range 8.0-22.0 mg/dl) and 5.2 mg/dl (normal range 0.7-1.4 mg/dl), respectively. The calculation using the modified diet for renal disease (MDRD) showed that the glomerular filtration rate (GFR) was 11 ml/min/1.73 m2. Urinary tract infection was suspected. The drug treatment was changed again to intravenous administration of cefepime (1 g/day) for the treatment of urinary tract infection and previously identified lesions. On the third day of the cefepime administration, the patient complained of severe abdominal pain. Abdominal sonography and computed tomography (CT) were performed, but they revealed no particular findings. On the fourth day, the patient began falling into a state of stupor, was unable to recognize family members, groaned, constantly complained of abdominal pain, and refused meals and drug administration. On the fifth day of the administration, aphasia and complete disorientation occurred. The patient's biometric signs were stable, and blood chemistry was found to be similar to previous findings. On the sixth day of the administration, neurotoxicity due to cefepime was suspected and administration of antibiotics was stopped, and electroencephalography was performed. As epilepsy and a triphasic wave were observed, the administration of an anticonvulsant was initiated (Fig. 1). On the fifth day of the conservative treatment that was performed after the discontinuation of the administration of antibiotics, the patient's consciousness and the findings from the electroencephalography improved. On the sixth day the electroencephalography findings were completely normal and the administration of the anticonvulsant was stopped. The patient recovered without particular sequelae.
Case 2
A 77-year-old female patient had a cold for 2 weeks and could not eat nearly anything before presentation to the authors' hospital. The patient had lost 20% body weight and systemic weakness was observed, and was hospitalized via the Department of Rehabilitation at Konkuk University Medical Center. The patient had a history of chronic renal dysfunction and was under a follow-up to determine the disease course. Blood chemistry analyses performed on the first day of hospitalization revealed an increased white blood cell (WBC) count (13.03×103/µl; normal range 4-10/µl) and urinalysis revealed pyuria. The patient also exhibited febricity. As urinary tract infection was suspected, ceftriaxone, as an empirical antibiotic, was intravenously administered (2 g/day). On the first day of hospitalization, the BUN and creatinine levels increased to 27.1 mg/dl (normal range 8.0-22.0 mg/dl) and 1.9 mg/dl (normal range 0.7-1.4 mg/dl), respectively, and the calculations using MDRD showed that the GFR was 26 ml/min/1.73 m2. After one week, the WBC level was 15.57×103/µl. The results of the urinary tract test had not improved and urine culture for antibiotic susceptibility indicated the presence of bacteria resistant to ampicillin/clavulanic acid and cefoxitin/ciprofl oxacin. Suspecting the elaboration of β-lactamase by the resistant bacteria as the cause, the drug regimen was changed to intravenous administration of cefepime (1 g/day). On the second day of cefepine use, the patient's consciousness decreased with aphasia and a muscle clamp. Cefepime administration was stopped with follow-up. Brain CT showed no particular findings, and the electroencephalography revealed a triphasic wave (Fig. 1). Hemodialysis was performed. Three days later, the patient's consciousness returned but the aphasia continued. The patient subsequently experienced cycles of systemic improvement and aggravation. One month later, the patient died of a pneumonia complication.
DISCUSSION
Epilepsy related to β-lactam antibiotics can occur due to competitive antagonistic actions for gamma-aminobutyric acid. This is attributed to the structural similarity between gamma-aminobutyric acid and a neurotransmitter.5 The mechanism remains unclear. The mortality resulting from neurotoxicity related to cefepime has been reported to be higher than that from other β-lactam antibiotics.6
Cefepime is excreted mostly via glomerular filtration, and its neurotoxicity has been reported mainly in patients with renal insufficiency.2,3 Although cases of cefepine-related neurotoxicity are sporadically reported, the drug's empirical incidence rate has been reported as about 3%,7 although this figure may be low.8,9
Previous studies reported that the time to the onset of symptoms (e.g., decrease in consciousness, epilepsy, aphasia, convulsion, and coma) from the administration of cefepime averages 5 days (range 1-10 days).2,8 The two patients in this case report complained of neurological adverse events 2 and 4 days after the administration of cefepime, and their neurological symptoms included decrease in consciousness, disorientation, aphasia, convulsion, and anxiety. Both patients displayed a triphasic wave in electroencephalography, which suggested metabolic encephalopathy. The clear association with the antibiotic treatment from the viewpoint of the timing, and both clinical and electroencephalographic improvements after the discontinuation of the antibiotics (case 1), strongly suggest cefepime-related neurolotoxical adverse events.
Diagnosis of such cases can be delayed by the presence of multiple factors, including old age, severe infection, multiple comorbidities, administration of multiple drugs, and the level of aggravation of medical diseases. Although the earliest discontinuation of the administration can be beneficial once a diagnosis was made,8-10 one of our patients died. Many cases of adverse events related to safety and mortality have been reported,2,3,6-8 and thus, care must be taken.
In conclusion, we report on our experience with two cases of neurological adverse events caused by cefepime. More careful clinical consideration is required for patients who are elderly and have renal insufficiency, and studies with various approaches to safety are required.