- Search
| Ann Rehabil Med > Volume 47(2); 2023 > Article |
|
Abstract
Objective
To evaluate treadmill backward walking training (BWT) effects on walking speed, balance, mobility, and walking endurance in children with cerebral palsy (CP).
Methods
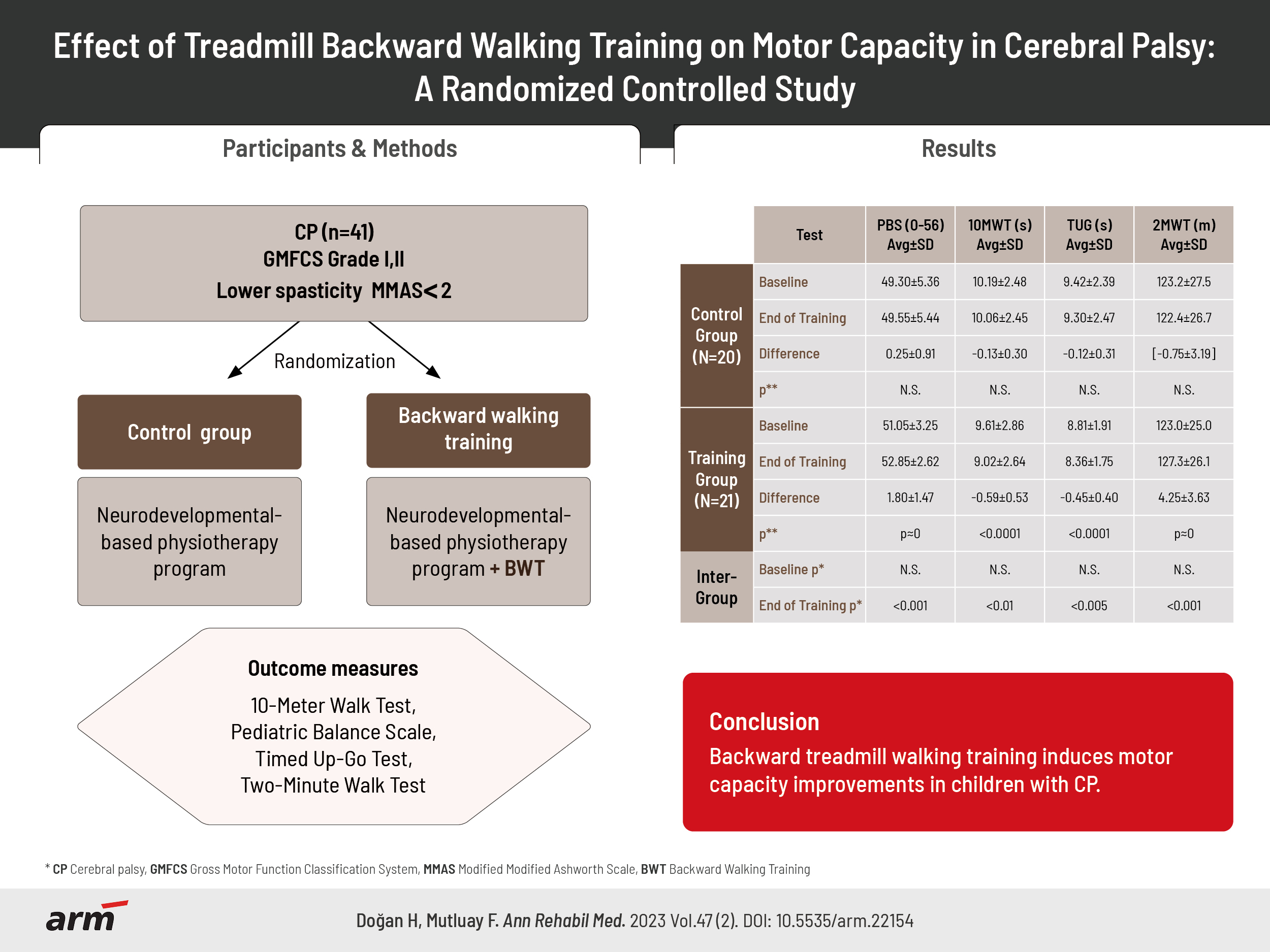
The study evaluated 41 children with CP (age, 6ŌĆō18; Gross Motor Function Classification System levels I and II). They were randomly allocated into control and BWT groups. BWT was applied (two sessions/week, 15 min/session for 8-week) to BWT group after the neurodevelopmental-based physiotherapy program routinely followed by all participants while the control group did not receive BWT. 10-Meter Walk Test (10MWT), Pediatric Balance Scale (PBS), Timed Up and Go Test (TUG), and Two-Minute Walk Test (2MWT) were selected as outcome measures for assessing walking speed, balance, mobility and endurance respectively.
Results
In BWG, 2MWT distance (3.5%), PBS (3.5%) increased significantly, and TUG decreased by 5.1% (all p<0.001) after training, 10MWT was shorter by 6.1% for BWG, corresponding to 7.4% faster walking speed (p<0.01). Control group assessment variations were stationary and not statistically significant.
Cerebral palsy (CP) is a group of permanent diseases caused by a nonprogressive lesion in the developing brain, where posture and movement disorders lead to activity limitations [1]. Balance and gait disorders, which are common in CP, cause loss of independence in activities of daily living and psychosocial negative effects by restricting the functionality of the child [2-4].
Increasing functionality is the ultimate goal in CP rehabilitation, thus improving motor capacity is of paramount importance [4,5]. Motor capacity includes walking speed, walking endurance, mobility, and balance factors. Clinical rehabilitation programs using forward, backward, sideways, and cross walking training on various surfaces are known to improve balance and kinematic gait parameters [6-10]. In neuro-rehabilitation, backward walking training (BWT) is an alternative method which has been recently applied for improving motor capacity. Backward walking, although using the same rhythm cycle, is not thought of as a simple reversal of forward walking since it requires much higher physiological, sensory and perceptual effort [11-13]. BWT has been reported to ameliorate balance, postural control, walking parameters, and motor function in studies on the healthy population [9,14], stroke patients [10] and the elderly [5] in the literature. Moreover, it may have biomechanical and physiological advantages over forward walking, further enhancing lower extremity muscle strength [15] and cardiopulmonary response [7].
While forward walking training (FWT) on treadmill for children with CP has been investigated for more than three decades with moderate improvements in gait speed and endurance [16], recent investigations have shown that BWT improves gross motor skills, balance, and walking parameters in children with CP [17-24]. These studies focused mainly on BWT effects on balance and gait parameters using specially designed non-standard (proprietary) test and training equipment, while its impact on mobility and walking endurance was not examined. However, current knowledge in neurorehabilitation confirmed by our experience shows that improvements in motor skills in neurological patients do not reflect on walking endurance at the same rate. Therefore, the main purpose of our study was to determine whether BWT improves walking endurance as well as balance and mobility in children with CP using only nonproprietary and easily accessible clinical assessment and evaluation tools which may be used in simple clinical settings.
Fifty-five children satisfying the study inclusion criteria specified in the next paragraph were selected as candidates from those attending regularly rehabilitation sessions in a private outpatient rehabilitation center. Seven candidates were dropped due to exclusion criteria described below; those remaining 48 children were invited and all willingly accepted to participate in the study. The willing participants were randomly divided into equalsized control and training groups by using sealed envelopes, shuffled and selected by a third person unrelated to the study. Twenty-four children started their backward walking augmented training program in the training group; one child could not adapt to backward walking and dropped from the study, while two children attended the training sessions irregularly and were not included in the final evaluation. Twenty children out of 24 included in the control group duly attended their pre- and posttraining evaluations. Control group children and their parents were not informed about any of the additional exercices received by the training group participants. The physiotherapist in charge of BWT was not blinded to the BWT group participants since he was fully in charge of BWT sessions. The flowchart of the study is given in Fig. 1.
Children aged 6ŌĆō18 with a medical report diagnosis with CP, classified at I or II levels for Gross Motor Function Classification System (GMFCS) [25] whose lower extremity spasticity assessed scores not exceeding two according to the Modified Modified Ashworth Scale (MMAS) [26] and who could understand and apply verbal commands and instructions were selected as candidates for the study.
Children with active epileptic seizures, mental and cognitive problems, visual or auditory defects, fixed deformities in their lower extremities, or an history of surgical intervention in the lower extremity musculoskeletal system in the last 6 months as reported in their medical files or specifically signalled by their pediatric neurologist were dropped from the selected candidates list as were those who have undergone botulinum toxin injection or using spasticity medications.
This is a simple measure of spasticity; it quantifies the resistance encountered during passive soft-tissue stretching [26]; the scale ranges from 0 (non-spastic) to 4 (rigid).
This is 5-level (IŌĆōV) classificationthat differentiates children with CP based on theirgross motorabilities, function, and mobility limitations [25]. GMFCS levels are normatively linked [27] with the Gross Motor Function Measure (GMFM) which is a finer graded instrument measuring the spectrum of gross motor activities in five dimensions: lying and rolling, sitting, crawling, standing (dimension D), and walking/running (dimension E). The original 88-item GMFM-88 for healthy children has been simplified to GMFM-66 version for children with CP; both have identical dimensions D (13-items) and E (24-items) which are separately scored in percentage points. GMFM has not been evaluated in our study but referred to in the discussion section for comparing our results with those reported in the literature.
The research protocol was designed according to the CONSORT guidelines (see CONSORT checklist). This study was conducted in accordance with the Helsinki Declaration Rules. Detailed verbal and written information was given to the individuals and their families and an informed consent form was required to be signed by the parents of each study participant. The study was approved on November 14, 2018 by the Institutional Review Board (IRB) of Istanbul Medipol University for NonInvasive Clinical Research (IRB no. 604) and recorded in ClinicalTrials (no. NCT04136678).
This is a randomized controlled clinical study. Socio-demographic and clinical characteristics of the participating children were recorded in their evaluation form before initiating the training sessions.
All study participants continued their 2 days/week, 30 minutes duration sessions of neurodevelopmental-based physiotherapy which they regularly attended before the initiation of the study. Exercises for these sessions were planned and applied according to the needs of the participants to facilitate balance and corrective reactions, strengthen weak trunk muscles, and improve postural control. These conventional training sessions were supervised and applied by different physiotherapists who were fully blinded to the study and its participating children.
Training group members, after their regular physiotherapy session, rested for 15 minutes and then received BWT applied for 15 minutes on a treadmill. The additional BWT was applied for a period of 8 weeks. Control group did not receive any supplemental training.
For safety, each BWT training session was started slowly: children were invited to walk at their lowest speed for one minute to adapt to the treadmill as a warm-up. After the warm-up, treadmill speed was imposed at 1.0 m/s; children were encouraged to increase their speed when they felt comfortable. Whenever a child wanted to backwalk faster, the treadmill was gradually accelerated up to a maximum of 1.6 m/s with incremental steps of 0.1 or 0.2 m/s depending on the childsŌĆÖ wishes and childsŌĆÖ apparent level of fatigue judged by the physiotherapist. Children were repeatedly instructed to hold onto the handles if feeling uncomfortable or losing balance while walking on the treadmill. They have been told to rest whenever they wanted and keep walking whenever they felt they could continue; the time was paused each time the participant needed a break and rest. The physiotherapist never encouraged children to reach the study maximal walking speed limit or insisted on the completion of a 15 minutes session length.
Performance assessments were completed at the beginning of the study (baseline data) and repeated for both groups at the end of the 8-week long training period by the same physiotherapist supervising BWT sessions. All outcome results were statistically analyzed and compared after the final evaluation by a bio-statistician blinded to the study.
Our study assessed the balance of the participants using Pediatric Balance Scale (PBS). The scale quantifies balance impairments in daily life activities by scoring 0ŌĆō4 points for the ability to maintain balance while sitting and standing, during 360 degree turns and transfers between juxtaposed chairs, when picking objects from the floor, placing an alternate feet on a stool and leaning forward in a standing position. The total PBS score were obtained by summing all 14 test item scores (max score, 56), high scores indicating better balance [15]. PBS has been found to be very strongly correlated with GMFM dimension D (rŌēł0.93) and E (rŌēł0.90) scores of GMFM-66 in a previous study [28].
The change in the covered distance during the test quantitatively evaluates the treatment impact on walking endurance. Two-Minute Walk Test (2MWT) was chosen as our primary evaluation criterion because it is considered valid and reliable especially in pediatric neurological patient population [16] and for normative data for healthy children is available [29,30]. In the 30-meter corridor where the test was performed, the starting and turning back lines were taped on the ground and the children were asked to walk the maximum distance they could walk between these lines in 2 minutes. The distance walked was recorded in meters with half-meter precision. The participants were asked to walk at their fastest but safe pace and were monitored closely by the physiotherapist for proper compliance with these instructions.
Timed Up and Go Test (TUG) was chosen as a measure of mobility and dynamic balance. It evaluates simultaneous components such as walking speed, postural control, mobility, and balance [31,32]. Children were asked to get up from a chair without armrests and walk a marked distance of 3 meters at normal speed, turn around and walk to sit back on the chair; the elapsed time was recorded in seconds with a chronometer and recorded with 100 ms precision.
10-Meter Walk Test (10MWT) evaluates the maximal achievable walking speed for a short period. The duration of walking between two colored strips (taped on the ground and spaced 10 meters apart) in a well-lit corridor at the fastest speed the child was willing to achieve safely was recorded with 50 ms precision. The test was repeated twice, as recommended [31], with the average of 2 completion times recorded for baseline evaluations before initiating training sessions; posttraining tests were performed only once due to operational limitations.
The mean┬▒standard deviation of the outcome measurements were calculated; their variation ranges were also identified. The compliance of the results with the normal (Gaussian) distribution was checked using the ShapiroŌĆōWilk statistic. Posttraining pairwise outcome differences were statistically analyzed using the StudentŌĆÖs t-test. Non-parametric intergroup differences were examined using chi-squared test.
All correlation investigations made used of PearsonŌĆÖs linear correlation method. Statistical analysis significance level was chosen as p<0.05.
Demographic and clinical information of the participants are given in Table 1. There were no significant differences in weight, height, and body mass index (BMI) between the control and training groups. Almost twothirds of the children were at the spastic hemiparetic type CP level. No sex-related statistically significant differences were detected in their baseline assessment data.
During BW T sessions, very few (only 4) children achieved the maximal target speed (1.6 m/s) and this for only a short time (around one half minute). One child could not adapt to BWT and dropped from the study.
Statistics for all outcome measurement results are shown in Table 2. There were no significant differences between groups in any of the outcome measures evaluated before starting the training sessions. No sex-related statistically significant differences of outcome variations were found. All evaluations were found normally (Gaussian) distributed according to ShapiroŌĆōWilk statistics.
Highly significant improvements in all outcome measures evaluated for the BWT group have been observed, while control group member assessments remained almost stationary during the training period. Training group 2MWT distance and PBS scores improved by 3.5% and their mean TUG Test time was 5.1% shorter after BWT. Moreover, their 10MWT duration was reduced by 6.1%, corresponding to an average relative short-distance walking speed improvement of 7.4%. The corresponding effect sizes, as evaluated with CohenŌĆÖs-d measure, have been computed as d=0.61 (PBS), d=0.26 (TUG), d=0.23 (10MWT) and d=0.18 (2MWT).
The eventuality of the interdependence of the outcome assessments of the study and their posttraining variations was investigated using PearsonŌĆÖs linear correlation analysis. Weak correlations were detected between TUG improvements with those assessed for PBS (r=-0.39, p<0.05) and measured for 10MWT (r=0.41, p<0.05). In addition, no dependence on BMI, MMAS and GMFCS levels were identified. Moreover, all posttraining outcome variations were found independent of their baseline assessed values.
This study was planned to examine the effects of treadmill BWT on walking speed, walking endurance, mobility, and balance in children with CP as evaluated with the outcome measures we selected to assess these factors affecting motor capacity. Our 8-week-long supplemental BWT program improved significantly all measured outcome assessments. These improvements were independent from the participants clinical characteristics and baseline performance levels.
The results suggest that BWT, as an unusual and different practice, is effective in improving walking endurance, balance, and walking speed, as it works the musculoskeletal system differently, requiring more balance, coordination skills, and attention.
Improvements in mobility (TUG) correlated with those in balance (PBS) and short-time walking speed (10MWT). Since mobility involves both balance and ambulatory speed factors, this observed interdependence should be expected. On the other hand, the independence of balance (PBS) improvements from those for walking speed (10MWT) and walking endurance (2MWT) may point to a dual but independent effect of BWT on balance and ambulatory performance.
The independence of observed training-induced outcome variations from their baseline assessment scores as well as from MMAS and GMFCS levels suggests that the progress achieved in the training group with BWT intervention is uniform, i.e., does not depend on the childŌĆÖs neurological impairment severity and therefore is not limited to children at predetermined impairment levels.
The effect of FWT on the treadmill on children with CP have been investigated for more than three decades; a comprehensive review of pertinent quantitative research. In 2019, Witherspoon et al. [16] reported significant moderate improvements in gait speed and endurance. Although BWT benefit in neurorehabilitation was established knowledge, it has only been recently used as an alternative to FWT in children with CP. The first identified BWT pilot study (without a control group) by Kim et al. [23] in 2013 with 12 participants aged 5ŌĆō15 used video-based real-time gait analysis in which moderate improvements on gait speed and balance (in GMFM-88 dimensions D and E) were claimed. Later, in a series of studies by a separate research group, the effect of BWT was investigated for children with CP aged 5ŌĆō7 at GMFCS levels IIŌĆōIII [18,19]; 5ŌĆō9 at IŌĆōIII [23]; 7ŌĆō11 at IIŌĆōIII [20,21]; and 10ŌĆō14 at IŌĆōII [17,22] concentrating on gait parameters including speed as well as on static and dynamic balance outcomes. This latter series of studies had 12 to 30 participants each with half of them assigned to control groups and used an additional body weight support system when appropriate. BWT was applied 3 times per week for 12 weeks, except only for 6 weeks in [18,19]. A metareview of their findings can be found in [5]; they observed dramatic (>50%) improvement in gait speed which they attributed mostly to improved strength and ~7% average amelioration of balance parameters. Only one study [17] compared backward walking (on the ground without using a treadmill) with FWT with no significant advantage of one over the other method. In all these past studies, specially designed proprietary systems were utilized to analyze gait speed and structural balance, none made use of commonly accepted clinical measures such as 10MWT, 2MWT, or TUG.
Although we found the progress seen after BWT statistically highly significant, the relative ameliorations achieved were small, all around 5% corresponding to small CohenŌĆÖs-d effect sizes (around d=0.2) except for PBS with a medium size effect variation (d=0.61). The effect reported in previous work were larger: the gait speed improvement reported in previous studies for 10MWT exceeded 50% in [17,20,24]; although with very high dispersion and was reported as 7.6% by Kim et al. [23], 2013 similar to our measured value of 6.1%. However, in these studies the participants walked at a much slower rate ŌĆö by four times in [17,20,24] and twice slower in [23] ŌĆö and their speed measurement method was different (selfselected comfortable rate vs. an encouraged competitive one in this study 10MWT). Likewise, only two studies [17,23] reported clinically reproducible (GMFM-88) balance improvements of 6%ŌĆō8% and 8%ŌĆō15% in dimensions D and E respectively. We observed only a ~3.5% score increase using PBS, which is over 90% correlated, thus comparable, with GMFM-88. One must note that our BWT program regimen was lighter than that applied in previous studies: 2 sessions per week vs. 3 sessions per week; 15 minutes per session vs. 15ŌĆō25 minutes per session; 8 weeks duration vs. 12 weeks duration. This considerable difference in training time may partially explain the relatively smaller effect we achieved with BWT compared to previously reported values. Although the effect size, as evaluated with CohenŌĆÖs-d measure, may be judged medium for balance improvement (d>0.6) and weak for those in mobility, walking speed and walking endurance (dŌēł0.2), we must conclude the progress we observed, although significant, is below clinically significant thresholds [33,34]. Longer period or more intensive application of BWT may improve the impact on clinical outcomes.
This study contribution may be summarized as follows. First, although our exercise training methods were mostly similar to those applied in previous studies, unlike them, we used only nonproprietary and easily reproducible clinical scales for evaluating eventual progress; this may be used in further studies in a clinical setting to easily track improvements in motor capacity. Second, this study is the first to examine the effect of BWT on walking endurance. Finally, BWT effect on mobility was also investigated and it is the first report on this motor capacity factor.
BWT group performed both classical and BWT exercises from which one might suspect that increased total training time leads to better performance; however, BWT itself is clearly the principal effective factor for the progress measured since the progress due to classical training by itself was almost imperceptible.
Due to scheduling difficulties, follow-up assessments of the children who completed the full training program were not possible, thus we could not verify long-term preservation of the improvements observed.
It is possible that the positive impact of backward walking to be amplified if the method is applied for a longer period; this may especially be valid for improving walking endurance since endurance enhancement is usually considered a long-term objective in conventional exercise training. However, if the associated burden of repeated use of a treadmill is an operational handicap, we suggest adding backward walking activities on the ground in the conventional physiotherapy programs for children with CP. Walking backwards requires greater reliance on neuromuscular control, proprioceptive inputs, and protective reflexes due to the elimination of visual cues [13]; thus, for the safety of the child, it is important to take adequate precautions when encouraging backward walking during physiotherapy sessions or supervised games. If BWT effects can sustainably be increased over time, their contribution on to improving the quality of life may become significant.
In conclusion, BWT for children with CP has significant, albeit small, positive effects on walking speed, walking endurance, mobility, and balance. Improvements are observed to be independent from the disability level of the patients.
AUTHOR CONTRIBUTION
Conceptualization: Do─¤an H, Mutluay F. Methodology: Do─¤an H, Mutluay F. Formal analysis: Do─¤an H, Mutluay F. Writing ŌĆō original draft: Do─¤an H, Mutluay F. Writing ŌĆō review and editing: Do─¤an H, Mutluay F. Approval of final manuscript: all authors.
ACKNOWLEDGMENTS
The researchers wish to acknowledge our gratitude to the children, their parents, our volunteering colleagues, and the rehabilitation institution.
Table┬Ā1.
Demographic and clinical information of the participating children with cerebral palsy
| Participant | Control group (n=20) | Training group (n=21) | p-valuea) | |
|---|---|---|---|---|
| Age (yr) | 10.80┬▒3.38 | 11.15┬▒3.65 | NS | |
| MaximumŌĆōminimum | 6ŌĆō18 | 6ŌĆō18 | ||
| Weight (kg) | 38.0┬▒16.9 | 38.8┬▒16.5 | NS | |
| Height (cm) | 140.3┬▒20.4 | 144.3┬▒22.3 | NS | |
| Body mass index (kg/m2) | 18.32┬▒3.98 | 18.04┬▒4.27 | NS | |
| Sex | NSb) | |||
| Male | 11 (55.0) | 10 (47.6) | ||
| Female | 9 (45.0) | 11 (52.4) | ||
| Type of cerebral palsy | NSb) | |||
| Spastic | 19 (95.0) | 19 (90.5) | ||
| Ataxic | 1 (5.0) | 2 (9.5) | ||
| Involvement of spastic type cerebral palsy | NSb) | |||
| Hemiparesis | 17 (85.0) | 14 (66.7) | ||
| Diplegia | 2 (10.0) | 3 (14.3) | ||
| Quadriparesis | 0 | 2 (9.5) | ||
| Modified Modified Ashworth Scale | NSb) | |||
| Score 0 (not spastic) | 1 (5.0) | 2 (9.5) | ||
| Score 1 (mild) | 13 (65.0) | 12 (57.1) | ||
| Score 2 (moderate) | 6 (30.0) | 7 (33.3) | ||
| Gross Motor Function Classification System | NSb) | |||
| Level I | 15 (75.0) | 14 (66.7) | ||
| Level II | 5 (25.0) | 7 (33.3) | ||
Table┬Ā2.
Analysis of baseline and posttraining outcome evaluations in children with cerebral palsy
| Test | PBS (0ŌĆō56) | 10MWT (s) | TUG (s) | 2MWT (min) | |
|---|---|---|---|---|---|
| Control group (n=20) | |||||
| Baseline | 49.30┬▒5.36 | 10.19┬▒2.48 | 9.42┬▒2.39 | 123.2┬▒27.5 | |
| End of training | 49.55┬▒5.44 | 10.06┬▒2.45 | 9.30┬▒2.47 | 122.4┬▒26.7 | |
| Difference | 0.25┬▒0.91 | -0.13┬▒0.30 | -0.12┬▒0.31 | -0.75┬▒3.19a) | |
| p-valueb) | NS | NS | NS | NS | |
| Training group (n=21) | |||||
| Baseline | 51.05┬▒3.25 | 9.61┬▒2.86 | 8.81┬▒1.91 | 123.0┬▒25.0 | |
| End of training | 52.85┬▒2.62 | 9.02┬▒2.64 | 8.36┬▒1.75 | 127.3┬▒26.1 | |
| Difference | 1.80┬▒1.47 | -0.59┬▒0.53 | -0.45┬▒0.40 | 4.25┬▒3.63 | |
| p-valueb) | Ōēł0 | <0.0001*** | <0.0001*** | Ōēł0 | |
| Intergroup | |||||
| Baseline p-valuec) | NS | NS | NS | NS | |
| End of training p-valuec) | <0.001*** | <0.01** | <0.005** | <0.001*** | |
REFERENCES
1. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M. A report : the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol 2007;49(Suppl 109): 8-14.

2. Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin JF. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther 2007;87:248-57.




3. Gage JR, Novacheck TF. An update on the treatment of gait problems in cerebral palsy. J Pediatr Orthop B 2001;10:265-74.


4. Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil 2011;90:137-49.


5. Elnahhas AM, Elshennawy S, Aly MG. Effects of backward gait training on balance, gross motor function, and gait in children with cerebral palsy: a systematic review. Clin Rehabil 2019;33:3-12.



6. Damiano DL, Wingert JR, Stanley CJ, Curatalo L. Contribution of hip joint proprioception to static and dynamic balance in cerebral palsy: a case control study. J Neuroeng Rehabil 2013;10:57.



7. Emara HA, El-Gohary TM, Al-Johany AA. Effect of body-weight suspension training versus treadmill training on gross motor abilities of children with spastic diplegic cerebral palsy. Eur J Phys Rehabil Med 2016;52:356-63.

8. Richards CL, Malouin F, Dumas F, Marcoux S, Lepage C, Menier C. Early and intensive treadmill locomotor training for young children with cerebral palsy: a feasibility study. Pediatr Phys Ther 1997;9:158-65.
9. Terblanche E, Page C, Kroff J, Venter RE. The effect of backward locomotion training on the body composition and cardiorespiratory fitness of young women. Int J Sports Med 2005;26:214-9.


10. Yang YR, Yen JG, Wang RY, Yen LL, Lieu FK. Gait outcomes after additional backward walking training in patients with stroke: a randomized controlled trial. Clin Rehabil 2005;19:264-73.



11. Flynn TW, Connery SM, Smutok MA, Zeballos RJ, Weisman IM. Comparison of cardiopulmonary responses to forward and backward walking and running. Med Sci Sports Exerc 1994;26:89-94.


12. Hoogkamer W, Meyns P, Duysens J. Steps forward in understanding backward gait: from basic circuits to rehabilitation. Exerc Sport Sci Rev 2014;42:23-9.

13. Kramer J. Backward walking: a cinematographic and electromyographic pilot study. Physiother Can 1981;33:77-86.
14. Threlkeld AJ, Horn TS, Wojtowicz G, Rooney JG, Shapiro R. Kinematics, ground reaction force, and muscle balance produced by backward running. J Orthop Sports Phys Ther 1989;11:56-63.


15. Franjoine MR, Gunther JS, Taylor MJ. Pediatric Balance Scale: a modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr Phys Ther 2003;15:114-28.


16. Witherspoon JW, Vasavada R, Logaraj RH, Waite M, Collins J, Shieh C, et al. Two-minute versus 6-minute walk distances during 6-minute walk test in neuromuscular disease: Is the 2-minute walk test an effective alternative to a 6-minute walk test? Eur J Paediatr Neurol 2019;23:165-70.



17. Abdel-Aziem AA, El-Basatiny HM. Effectiveness of backward walking training on walking ability in children with hemiparetic cerebral palsy: a randomized controlled trial. Clin Rehabil 2017;31:790-7.



18. Abdou R, El-Negamy E, Hendawy A. Impact of backward gait training on Mediolateral stability index in children with hemiparesis. J Med Sci Clin Res 2014;2:2042-9.
19. Hegazy RG. Impact of backward treadmill walking on balance in children with diplegic cerebral palsy. Int J Ther Rehabil Res 2017;6:141-7.

20. Ayoub HESAA. Forward versus backward body weight supported treadmill training on step symmetry in children with spastic diplegia. Int J Physiother Res 2016;4:1639-45.

21. Ayoub HESAA. Impact of body weight supported backward treadmill training on walking speed in children with spastic diplegia. Int J Physiother 2016;3:535-9.

22. El-Basatiny HM, Abdel-Aziem AA. Effect of backward walking training on postural balance in children with hemiparetic cerebral palsy: a randomized controlled study. Clin Rehabil 2015;29:457-67.



23. Kim SG, Ryu YU, Je HD, Jeong JH, Kim HD. Backward walking treadmill therapy can improve walking ability in children with spastic cerebral palsy: a pilot study. Int J Rehabil Res 2013;36:246-52.

24. Sanad DAM. Conditioning effects of backward treadmill training in children with spastic diplegic cerebral palsy. Int J Physiother Res 2017;5:2294-300.

25. Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol 2008;50:744-50.


26. Ansari NN, Naghdi S, Younesian P, Shayeghan M. Inter- and intrarater reliability of the Modified Modified Ashworth Scale in patients with knee extensor poststroke spasticity. Physiother Theory Pract 2008;24:205-13.


27. Hanna SE, Bartlett DJ, Rivard LM, Russell DJ. Reference curves for the Gross Motor Function Measure: percentiles for clinical description and tracking over time among children with cerebral palsy. Phys Ther 2008;88:596-607.




28. Yi SH, Hwang JH, Kim SJ, Kwon JY. Validity of pediatric balance scales in children with spastic cerebral palsy. Neuropediatrics 2012;43:307-13.


29. Bohannon RW, Wang YC, Bubela D, Gershon RC. Normative two-minute walk test distances for boys and girls 3 to 17 years of age. Phys Occup Ther Pediatr 2018;38:39-45.


30. Pin TW, Choi HL. Reliability, validity, and norms of the 2-min walk test in children with and without neuromuscular disorders aged 6-12. Disabil Rehabil 2018;40:1266-72.


31. Chrysagis N, Skordilis EK, Koutsouki D. Validity and clinical utility of functional assessments in children with cerebral palsy. Arch Phys Med Rehabil 2014;95:369-74.


32. Nicolini-Panisson RD, Donadio MV. Normative values for the Timed ŌĆśUp and GoŌĆÖ test in children and adolescents and validation for individuals with Down syndrome. Dev Med Child Neurol 2014;56:490-7.